Giant cell tumor
| Classification according to ICD-10 | |
|---|---|
| D16.- | Benign neoplasm of bone and articular cartilage |
| D48.0 | New formation of unsafe or unknown behavior in bones and articular cartilage |
| D48.1 | Formation of unsafe or unknown behavior in connective tissue and other soft tissue |
| ICD-10 online (WHO version 2019) | |
The giant cell tumor is a very rare and generally benign tumor of the soft tissue or bone , but it grows aggressively locally. In the bone it is also often referred to synonymously as osteoclastoma .
pathology
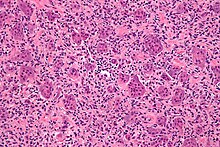
His name has the tumor because of its characteristic, the microscope visible large, multinucleated osteoclasts -like giant cells . The actual tumor cells, however, are the mesenchymal mononuclear fibroblast-like cells lying in between . However, the morphology of the cell nuclei of the mononuclear cells and that of the giant cell nuclei is very similar, which is histologically groundbreaking. The mononuclear cells typically have mutations in the histone H3 protein family (H3F3A) and produce large amounts of the RANK ligand , which activates the giant cells.
In addition to giant cell tumors, there are other tumors with a giant cell-like histological appearance, in which differentiation is sometimes difficult, but in which the cell nuclei of the proliferating mononuclear cells differ significantly from the appearance of the cell nuclei of osteoclast-like giant cells:
- Brown tumor in the context of hyperparathyroidism with irregularly distributed giant cell clusters mainly located around hemorrhages
- reparative giant cell granuloma , also with giant cell clusters in an irregular distribution around hemorrhages
- Chondroblastoma predominantly in the epiphyses before the end of growth
- Osteosarcoma as a malignant bone tumor in which, however, malignant cells with osteoid or bone formation must be present
Giant cell tumor of the bone
Giant cell tumors are found in about 3/4 of the cases on the extremities and in a quarter of the cases on the trunk. On the extremities, giant cell tumors are largely restricted to the epiphysis of the long tubular bones and are the most common benign bone tumors there, alongside the chondroblastoma . Due to their location in the epiphysis, they are almost always close to the joint. The most common location in half of all extremity giant cell tumors is the knee joint with the distal femoral epiphysis in 34% and the proximal tibial epiphysis in 29%.
However, the tumor is rare, with around nine new cases per 1 million population.
Young adults between the ages of 20 and 45 are particularly affected, women more often. Giant cell tumors of the bone make up about 5% of all benign bone tumors .
The disease often goes undetected for a long time, as even aggressive giant cell tumors grow only slowly and often cause no pain. The causes of the development of this disease are not yet clear.
Giant cell tumors of the bone rarely form daughter tumors in other parts of the body (1 - 2%). Most metastases are found in the lungs , where they often appear benign histologically. Metastases are found more frequently in younger patients and in primary tumors that are growing more aggressively radiologically, as well as after a relapse and in tumors that are located in the extremities ("axially"). Metastases elsewhere are very rare. Due to the benign behavior of lung metastases in particular, giant cell tumors of the bone are among the few benign metastatic tumors.
As a rule, however, the tumors grow locally and aggressively into the surrounding tissue. After the tumor has been removed, there is a high risk of about 25% for a relapse . Less than 1% of the tumors degenerate and form a malignant sarcoma , which is then usually extremely malignant, often has p53 suppression and an HRAS mutation, and has a poor prognosis.
treatment
In June 2013, Amgen received US Food and Drug Administration (FDA) approval for denosumab (XGEVA ® ) for the treatment of giant cell tumors of bone. Denosumab is the first FDA-approved drug for this rare disease. It is a human monoclonal antibody that binds to the RANK ligand produced by the tumor cells and thus neutralizes it. This eliminates the stimulation of the osteoclasts. In two approval studies, 136 of 190 patients showed partial or complete remission and 179 of 190 patients showed no further progression of the bone tumor. With the monthly dose of 120 mg, however, the risk of osteonecrosis of the jawbone is significantly increased and is 1% per year of treatment.
Web links
Individual evidence
- ↑ a b c Alphabetical directory for the ICD-10-WHO Version 2019, Volume 3. German Institute for Medical Documentation and Information (DIMDI), Cologne, 2019, p. 757.
- ^ A b c Edwin Choy, Francis J. Hornicek, Yen-Lin Chen, Daniel I. Rosenthal, Darcy A. Kerr: Case 26-2016: A 28-year-old woman with back pain and a lesion in the lumbar spine New England Journal of Medicine 2016, Volume 375, Issue 8, August 25, 2016, Pages 779-788, DOI: NEJMcpc1505482
- ↑ X. Niu, Q. Zhang, L. Hao, Y. Ding, Y. Li, H. Xu, W. Liu: Giant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institution. In: The Journal of Bone and Joint Surgery. American volume. Volume 94, Number 5, March 2012, pp. 461-467, ISSN 1535-1386 . doi: 10.2106 / JBJS.J.01922 . PMID 22398741 .
- ↑ FDA Approves Amgen's XGEVA ® (denosumab) For The Treatment Of Giant Cell Tumor Of Bone , press release June 13, 2013