Integrins
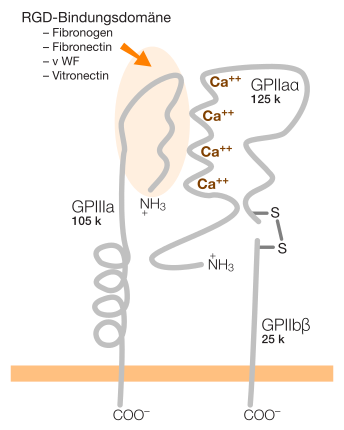

Integrins are protein molecules that occur in all animal cells with the exception of erythrocytes . They are permanently anchored in the cell membrane and cross the cell membrane. They count among the transmembrane proteins .
Overview
Integrins connect cells with other cells and with the extracellular matrix (ECM) . They are also important for the transmission of signals between cells and their surroundings. Integrins belong to the group of adhesion molecules and are receptors for extracellular proteins. At least three other proteins play an important role in cell-cell and cell-matrix interaction or communication - the cadherins , CAMs (cell adhesion molecules) and selectins .
The extracellular protein domain of these transmembrane proteins has binding sites with an RGD peptide, for example in fibronectin for αVβ3 and α5β1 integrin, or for “non-RGD proteins” such as intercellular adhesion molecules (ICAMs), collagens and laminin (in epithelial cells ).
Integrins are glycoproteins ; H. they have several sugar chains on their surface. In terms of structure, they are heterodimers , i.e. they consist of two different, interconnected glycoprotein chains. In humans, 24 different integrins can be built up from the previously known 18 alpha and 8 beta subunits. Other studies assume 19 alpha and 8 beta subunits, which form 25 integrin heterodimers. In addition, there are different isoforms for several alpha and beta subunits through alternative splicing.
There are different ways to subdivide integrins. A structural differentiation is based on the presence of an additional α-I domain in the alpha subunit. Nine of the 24 integrins have this additional domain. Integrins with the α-I domain bind to ligands only through this domain. All other integrins (without the α-I domain), however, bind ligands together with the alpha and beta subunits. Integrins with the α-I domain are only found in vertebrates, which is associated with the development of the internal skeleton and the adaptive immune system in vertebrates.
Integrins themselves have no enzymatic function and do not make a direct connection to the cytoskeleton . Instead, integrins interact with a variety of adapter proteins. Together with these adapters, integrins form focal adhesions (connection to actin filaments ) or hemidesmosomes (only α6β4 integrin; connection to intermediate filaments ).
Integrins play an important role in many processes within the body. You can e.g. B. bind viruses , enable the directed migration of white blood cells into foci of inflammation or mediate certain steps in blood clotting .
Activation and inactivation of integrins
The activity of integrins is subject to strict regulation. Physiologically this is z. B. important for αIIbβ3 integrin, which is found on the surface of platelets . Activation of this integrin leads to the binding of proteins in the surrounding blood plasma (e.g. fibronectin and fibrinogen) and thereby to the formation of a thrombus. While this is important in wound healing, incorrect, pathological activation leads to thrombosis.
In general, the activity of integrins is regulated externally by the presence of ligands or internally by cellular adapter proteins ("outside-in" and "inside-out" activation). Changes in activity are linked to conformational changes. In the inactive state, the head domains of both subunits point towards the cell membrane, which makes integrins appear bent or kinked ("bent state"). Integrin activation leads to the erection of the alpha and beta subunits ("extended state"). As activation progresses, the transmembrane domains of the two subunits separate and are ready for binding to other proteins and to the actin cytoskeleton (see figure). The two most important proteins for integrin activation are Talin and Kindlin.
Much less is known about integrin inactivation than about activation. There are adapter proteins that keep integrins inactive. In the case of activated integrins, on the other hand, Talin and Kindlin lose their bond to the integrin and thus allow the return to the inactive conformation. Another possibility of inactivation involves the removal of integrins from the cell membrane by means of endocytosis .
However, there are several exceptions to these points throughout the integrin family and many integrins have not yet been adequately explored.
Perception of mechanical and chemical signals
Integrins perceive chemical signals through binding to extracellular proteins of the ECM or through intracellular adapter proteins and post-translational modifications . In recent years, however, it has also been shown for many integrins that they can perceive mechanical signals. Integrins of the ECM associated extracellular to proteins and intracellular via adapter proteins such as talin and vinculin to the actin - cytoskeleton . Molecular simulations suggest that tensile force along this ECM-integrin-actin axis leads to a stronger separation of the alpha and beta subunits within the cell. As a result, traction promotes the active state of integrins (see above on activation and inactivation of integrins). For some integrins there is now experimental evidence for these model predictions. This has implications for some diseases such as cancer or fibrosis , which are accompanied by a stiffening of the ECM.
In addition to the influence of tensile force on the conformation of integrins, the binding to ligands is also directly influenced. For several integrin-ligand combinations it has been shown that tensile forces extend the life of the bond ("catch bond"). Normal chemical bonds, on the other hand, tend to break with increasing tensile stress ("slip bond"). Various other surface receptors show a similar behavior.
In addition, integrin-mediated focal adhesions are also sensitive to mechanical signals and contribute to the perception of mechanical signals by a cell. Through the link to the actin cytoskeleton, integrins are ultimately also connected to the cell nucleus and influence the localization of transcription factors and thus gene regulation .
Integrins and viruses
Many viruses use integrins for attachment and / or to be taken up into cells. The high number of integrins on the cell surface (up to 100,000 and more) and their rapid exchange by means of endocytosis (half-life on the cell surface less than 15 min) make integrins presumably attractive gateways for viruses to get into cells. Integrin-binding viruses include adenoviruses , human cytomegalovirus , Epstein-Barr virus , HIV-1 , foot-and-mouth disease virus , human papillomavirus , and Ebola virus . Viruses in general are not restricted to specific integrins and use both the RGD peptide and different peptide sequences to bind to integrins. In contrast to SARS-CoV, SARS-CoV-2 has developed an RGD peptide. Whether SARS-CoV-2 binds to integrins and uses them for infection has not yet been researched.
If one inhibits the type of integrin recognized by a certain virus, one can successfully suppress infections in the cell culture. At the organism level, however, protection against viral infection by inhibiting integrins has not yet been shown.
Integrin antagonists as drugs
Altering the bond between integrins and molecules that bind to them has now become an important target in the development of new drugs . Possible applications include a. in inflammatory diseases (e.g. Crohn's disease or ulcerative colitis ) or in oncology . However, the first integrin-related drug that was approved for the market was the anti-coagulant abciximab in 1994 .
- Natalizumab , an inhibitor of binding between the occurring on white blood cell integrin α4β1 (VLA-4 = eng . "Very late antigen-4") with the VCAM-1 ( engl . "Vascular cell adhesion molecule 1") and fibronectin, was already as drugs for the treatment approved for relapsing multiple sclerosis . With the atomic force microscope, the binding force of VLA4 / VCAM-1 receptors between living cells could be examined. Natalizumab has been associated with an increased risk of developing progressive multifocal leukoencephalopathy (PML).
- Vedolizumab is a humanized monoclonal antibody belonging to the integrin antagonist group and approved in the EU and the US for the treatment of adults with moderately to severely active ulcerative colitis or moderately to severely active Crohn's disease. Vedolizumab binds to α4β7 integrin.
- Lifitegrast , an inhibitor of the LFA-1 / ICAM-1 interaction, is used in the treatment of dry eye ( keratoconjunctivitis sicca ).
List of all integrins
| Surname | Synonyms | Occurrence | Ligands |
|---|---|---|---|
| α1 β1 | VLA-1 | Fibroblasts , osteoblasts | Collagen |
| α2 β1 | VLA-2 | Fibroblasts | Collagen |
| α3 β1 | VLA-3 | Epithelial cells | Laminin |
| α4 β1 | VLA-4 | VCAM, fibronectin | |
| α4β7 | VCAM | ||
| α5 β1 | VLA-5; Fibronectin receptor | Fibroblasts , endothelial cells | Fibronectin |
| α6 β1 | VLA-6; Laminin receptor | Epithelial cells | Laminin |
| α6β4 | Epithelial cells | Laminin | |
| α7 β1 | Muscle cells | Laminin | |
| α8 β1 | |||
| α9 β1 | lymphatic vessels | Tenascin, proteases , fibronectin | |
| α10 β1 | Collagen | ||
| α11 β1 | Collagen | ||
| αV β1 | TGFβ , vitronectin , fibronectin | ||
| αV β3 | Vitronectin receptor | Fibroblasts , endothelial cells | Vitronectin , osteopontin , fibronectin , fibrinogen |
| αIIb β3 | GPIIbIIIa; Fibrinogen receptor | Platelets | Fibrinogen , fibronectin |
| αV β5 | Fibroblasts , retinal pigment epithelium | Vitronectin , fibronectin | |
| αV β6 | Epithelial cells | TGFβ | |
| αV β8 | Nerve tissue | TGFβ | |
| αD β2 | ICAM | ||
| αL β2 | LFA-1 | ICAM | |
| αM β2 | Mac-1 | ICAM | |
| αX β2 | ICAM | ||
| αEβ7 | E-cadherin |
See also
Individual evidence
- ↑ Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion . Science 2007; 316 : 1148-53, PMID 17525329 .
- ↑ Hynes R. Integrins: bidirectional, allosteric signaling machines Cell 2002; 110 : 673-87, PMID 12297042 .
- ↑ Humphries MJ: Integrin structure . In: Biochem. Soc. Trans. . 28, No. 4, 2000, pp. 311-339. doi : 10.1042 / 0300-5127: 0280311 . PMID 10961914 .
- ↑ a b c d Michael Bachmann, Sampo Kukkurainen, Vesa P. Hytönen, Bernhard Wehrle-Haller: Cell Adhesion by Integrins . In: Physiological Reviews . tape 99 , no. 4 , October 1, 2019, ISSN 0031-9333 , p. 1655–1699 , doi : 10.1152 / physrev.00036.2018 .
- ↑ Mark S. Johnson, Ning Lu, Konstantin Denessiouk, Jyrki Heino, Donald Gullberg: Integrins during evolution: Evolutionary trees and model organisms . In: Biochimica et Biophysica Acta (BBA) - Biomembranes . tape 1788 , no. 4 , April 2009, p. 779–789 , doi : 10.1016 / j.bbamem.2008.12.013 ( elsevier.com [accessed April 30, 2020]).
- ↑ David A. Calderwood, Iain D. Campbell, David R. Critchley: Talins and kindlins: partners in integrin-mediated adhesion . In: Nature Reviews Molecular Cell Biology . tape 14 , no. 8 , August 2013, ISSN 1471-0072 , p. 503-517 , doi : 10.1038 / nrm3624 , PMID 23860236 , PMC 4116690 (free full text) - ( nature.com [accessed April 30, 2020]).
- ↑ Daniel Bouvard, Jeroen Pouwels, Nicola De Franceschi, Johanna Ivaska: integrin inactivators: balancing cellular functions in vitro and in vivo . In: Nature Reviews Molecular Cell Biology . tape 14 , no. 7 , July 2013, ISSN 1471-0072 , p. 430–442 , doi : 10.1038 / nrm3599 ( nature.com [accessed April 30, 2020]).
- ^ Pontus Nordenfelt, Hunter L. Elliott, Timothy A. Springer: Coordinated integrin activation by actin-dependent force during T-cell migration . In: Nature Communications . tape 7 , no. 1 , December 2016, ISSN 2041-1723 , p. 13119 , doi : 10.1038 / ncomms13119 , PMID 27721490 , PMC 5062559 (free full text) - ( nature.com [accessed April 30, 2020]).
- ↑ Michael Bachmann, Markus Schäfer, Vasyl V. Mykuliak, Marta Ripamonti, Lia Heiser: Induction of ligand promiscuity of αVβ3 integrin by mechanical force . In: Journal of Cell Science . March 19, 2020, ISSN 0021-9533 , p. jcs.242404 , doi : 10.1242 / jcs.242404 .
- ↑ Yunfeng Chen, Lining Ju, Muaz Rushdi, Chenghao Ge, Cheng Zhu: Receptor-mediated cell mechanosensing . In: Molecular Biology of the Cell . tape 28 , no. 23 , November 7, 2017, ISSN 1059-1524 , p. 3134-3155 , doi : 10.1091 / mbc.e17-04-0228 ( molbiolcell.org [accessed April 30, 2020]).
- ↑ ViralZone page. Accessed April 30, 2020 .
- ↑ Patrick T. Caswell, Jim C. Norman: Integrin Trafficking and the Control of Cell Migration: Integrin Trafficking and Cell Migration . In: Traffic . tape 7 , no. 1 , January 2006, p. 14–21 , doi : 10.1111 / j.1600-0854.2005.00362.x ( wiley.com [accessed April 30, 2020]).
- ↑ Hosni AM Hussein, Lia R. Walker, Usama M. Abdel-Raouf, Sayed A. Desouky, Abdel Khalek M. Montasser: Beyond RGD: virus interactions with integrins . In: Archives of Virology . tape 160 , no. November 11 , 2015, ISSN 0304-8608 , p. 2669–2681 , doi : 10.1007 / s00705-015-2579-8 ( springer.com [accessed April 30, 2020]).
- ↑ Christian JA Sigrist, Alan Bridge, Philippe Le Mercier: A potential role for integrins in host cell entry by SARS-CoV-2 . In: Antiviral Research . tape 177 , May 2020, p. 104759 , doi : 10.1016 / j.antiviral.2020.104759 ( elsevier.com [accessed April 30, 2020]).
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Tysabri .
- ^ Eibl RH and Benoit M: Molecular resolution of cell adhesion forces. . In: IEE Proc Nanobiotechnol. . 151, No. 3, 2004, pp. 128-132. doi : 10.1049 / ip-nbt: 20040707 . PMID 16475855 .
- ^ Eugene O. Major: Progressive Multifocal Leukoencephalopathy in Patients on Immunomodulatory Therapies . In: Annual Review of Medicine . tape 61 , no. 1 , February 2010, ISSN 0066-4219 , p. 35–47 , doi : 10.1146 / annurev.med.080708.082655 ( annualreviews.org [accessed April 30, 2020]).
- ↑ FDA approves Entyvio to treat ulcerative colitis and Crohn's disease , PM of the Food and Drug Administration (FDA) dated May 20, 2014, accessed October 21, 2014
- ↑ Public assessment report (EPAR) of the European Medicines Agency (EMA) on: Entyvio .
- ↑ Summary of the EPAR for the public , EPAR in German, accessed on October 21, 2014.
- ↑ Entyvio prescribing information , Red List, trade information services, accessed October 21, 2014.