Cadherine
| Cadherin-1; E-cadherin | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 728 aa | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene names | CDH1 ; Arc-1; CD324; CDHE; ECAD; LCAM; UVO | |
| External IDs | ||
| Occurrence | ||
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | mouse | |
| Entrez | 999 | 12550 |
| Ensemble | ENSG00000039068 | ENSMUSG00000000303 |
| UniProt | P12830 | Q4KML8 |
| Refseq (mRNA) | NM_004360 | NM_009864 |
| Refseq (protein) | NP_004351 | NP_033994 |
| Gene locus | Chr 16: 67.33 - 67.43 Mb | Chr 8: 109.49 - 109.56 Mb |
| PubMed search | 999 |
12550
|
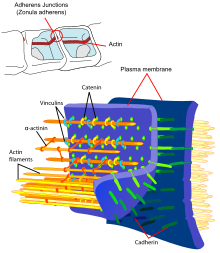
Cadherins (from English calcium adhering , like Ca-adherine ) are transmembrane glycoproteins from the group of adhesion proteins that are dependent on calcium ions (Ca 2+ ) . They occur in desmosomes and adherens junctions and cause cell contacts in various tissues. The cadherins play a role in stabilizing cell-cell contacts , embryonic morphogenesis , maintaining cell polarity and signal transduction .
With the help of cryoelectron tomography , it was possible to prove that the stabilization is carried out by thread-like adhesion proteins that protrude from the cell membranes and interlock.
Structure and subdivision
From the superfamily of cadherins, over 300 proteins are currently known in vertebrates alone . To date, more than 80 cadherins have been identified in humans. Common to all cadherins are several extracellular cadherin domains (ECs). An EC is about 100 amino acids long, evolutionarily very conserved and has negatively charged sequence motifs which mediate calcium ion-dependent, homophilic bonds. These ECs are repeated in tandem between 5 and 34 times over short linker sequences approx. Ten amino acids long, the ECs being numbered consecutively starting at the N-terminal end. Based on this number of ECs, but also on the cytoplasmic domain and the size of a cadherin as well as on gene clusters, the superfamily of cadherins is divided into seven groups:
| Subtype | Examples |
|---|---|
| classic Cadherine Type I. | E- and N-cadherins |
| classic cadherine type II | VE-Cadherine |
| desmosomal cadherins | Desmocolline, Desmogleine |
| Protocadherins | α-, β- and γ-protocadherins |
| Cadherin-like signal proteins | FAT, Daschous, Flamingo |
| Protein kinase cadherins | k. A. |
| 7 transmembrane cadherins | k. A. |
The most important and best studied representatives are the classic cadherins, such as E- and N-cadherins, as well as P (planzetare) and VE-cadherins. These also play an important role in malignant progression. They form cis-dimers in parallel in the cell. In this conformation, they are able to form a trans dimerization with an identical cadherin dimer in the opposite cell and thus bridge the extracellular space between the two cells. Classic cadherins are made up of five extracellular domains, with the binding sites of calcium ions between the individual domains, which are of fundamental importance for the adhesive function of the cadherins. The interaction of cadherin molecules on opposing cells (trans interaction) comes about through the N-terminal domains of the cells. These bonds are normally protein-specific (homophilic), i.e. an E-cadherin in one cell can only bind to an E-cadherin in another cell, but not e.g. B. an N-cadherin. However, heterophilic interactions of different cadherin subtypes are also rarely observed.
Non-classical cadherins include desmocollin ( mainly found in the skin in desmosomes), desmoglein ( mainly found in the skin in desmosomes), T-cadherin ( mainly in neurons and muscles).
E-cadherin
E-cadherin, one of the classic cadherins, which occurs mainly in the epithelia, is the best-studied cadherin and is regarded as the prototype molecule for the entire cadherin subfamily. It has 5 ECs in the extracellular domain, a transmembrane domain and an intracellular domain, which binds p120-catenin and β-catenin . This intracellular domain has a strongly phosphorylated region, which is essential for the binding of β-catenin (and thus for the function of E-cadherin).
The human E-cadherin gene is located on chromosome 16q22.1, the coding region consists of 2652 base pairs.
N-cadherin
N-cadherin (neuronal cadherin) corresponds in its structure to a classic cadherin and was isolated by Grunwald in 1982 as a 130 kDa molecule from chicken retina. It plays an important role in embryonic development as N-cadherin is formed in the mesoderm and notochord in the early embryonic stage. In the late embryonic stage, on the other hand, it is detectable in the nerve tissue, heart, skeletal muscle and in the lens.
In the adult organism, it is formed in nerve tissue and the retina, among other things. N-cadherin stimulates the migration and invasion of cells via cell-cell contacts and, together with the highly expressed E-cadherin, suppresses cancer formation in small quantities.
VE cadherin
Vascular-endothelial cadherin (VE-cadherin) is a special representative of the classic cadherin, which occurs in contact points of adjacent endothelial cells. In contrast to those of the epithelial cells, the contact points of endothelial cells do not have desmosomes. In endothelial contact points, VE-cadherin performs the function of classic cadherins of adherent contact points (“adherens junctions”) as well as desmosomal cadherins. The cytoplasmic domain binds, like other classic cadherins, β-catenin, which in turn regulates the actin cytoskeleton via α-catenin.
More than 80 different members of the cadherin family are known in the human organism. Of these, more than 30 cadherins have been identified in the developing human brain. Each cadherin has an expression pattern that is specific and determined by the synthesis region and the level of development of the organism.
Protocadherin GammaC3
By expression of the gamma -Protocadherin-A3- EGFP - fusion protein in cultured primary hippocampal neurons colocalization was observed after counterstaining with the synaptic marker synaptophysin. Also, Northern blot analyzes of total RNA from various tissues of adult mice showed the highest expression (approximately 4.8 kb) in the central nervous system. However, a lower level of expression was also observed in the lungs and, after prolonged exposure, also in the heart and kidneys. In contrast to other isoforms of the gamma protocadherins, which are mainly expressed during embryogenesis, the gamma protocadherin isoform C5 shows an increased postnatal expression in the brain, which coincides with the peak of synaptogenesis. In addition, a strong expression in the olfactory bulb , corpus striatum , dentate gyrus , the CA1 region of the hippocampus, layers I and II of the cortex cerebri and the molecular layer of the cerebellum could be shown. In addition, a strong expression of the isoform was detected in the glomeruli of the olfactory bulb.
A homophilic interaction between two identical gamma protocadherin isoforms was demonstrated which were transgenically expressed in cadherin-free K562 leukemia cells. At the same time it could be shown that this interaction takes place independently of calcium, in contrast to the N-cadherins. The cloning of chimeric gamma protocadherins also suggested the dependence of the homophilic interaction on the surface protein domains 2 and 3, which is a further difference from the N-cadherins, whose homophilic interaction is mediated via domain 1. In addition to a quantitative analysis of the cell aggregation, the highly specific, homophilic interaction was also illustrated by qualitative confocal microscopic analyzes after mixing isoform-expressing cells labeled in red and green.
In addition to this trans interaction, a highly promiscuous or unspecific formation of tetramers from a wide variety of isoforms was demonstrated. In a next step it could also be demonstrated that the promiscuous tetramer formation on the cell membrane is associated with a highly specific trans interaction between cells that have the same formation of tetramers in their membrane. The formation of the interaction between these cells also gradually decreases with the inequality of the tetramers formed . In summary, it can be said that the differential expression of the 22 isoforms 224 (234.256) gives rise to different possibilities for tetramer formation. If these tetramers have an identical composition, a highly specific cell-cell recognition is mediated. The detection of intracellular cis multimer formation of the gamma protocadherin isoforms with those of the alpha protocadherins also multiplies the number of possible specific cell-cell interactions.
Signal transduction
Cadherin-mediated cell adhesion is a dynamic process. This process depends on the topology and the differentiation stage of the cell, but it also has an effect on them. This means that cadherins are not only adhesion molecules, but also important signal molecules. The transmission of extracellular signals into the cell is brought about by the cytoplasmic cadherin domain.
Catenins
Catenins are cytoplasmic proteins for which an association with classical cadherins was first described. The catenin family includes α- and β-catenin, γ-catenin (plakoglobin) and δ-catenin (p120-catenin). In the intracellular space, catenins form the regulatory switching point between the transmembrane cadherins and the actin filaments of the cytoskeleton. The most important catenin in the development of cancer is β-catenin, which also plays a central role in the WNT signaling pathway. β-catenin binds both to the cytoplasmic domain of classic cadherins and to α-catenin, an important regulator of the actin cytoskeleton. γ-catenin is identical to plakoglobin, a protein which was first isolated from desmosomes. Like β-catenin, γ-catenin also binds to both the cytoplasmic cadherin domain and to α-catenin. Another protein associated with the cytoplasmic domain of classic cadherins is p120-catenin. p120-catenin modulates the effect of cadherins by regulating the cadherin turnover on the cell surface and, by influencing RhoA, Rac and Cdc42, also the dynamics of the cytoskeleton.
Cadherins and Carcinoma Progression
E-cadherin plays a prominent role in the progression of malignant tumors, as it is the essential epithelial cadherin. E-cadherin is the product of the CDH1 gene. In the course of embryonic development, CDH1 is already expressed in the blastomer stage. This gene product is involved in the compaction of the blastomeres. In the further course, E-cadherin is found in all epithelia, regardless of whether these arise from ecto-, meso- or endoderm. In the majority of human carcinomas, the loss of E-cadherin is due to a reduced transcription of the CDH1 gene due to promoter methylation or an altered regulation by transcription factors. In some types of tumors, E-cadherin is also inactivated by sequence mutation of the CDH1 gene. These are mainly stomach and breast cancers. Inactivating mutations of CDH1 are mainly found in tumors in which the tumor cells spread diffusely and largely individually. As with other tumor suppressor genes, most CDH1 mutations lead to premature chain termination or the loss of larger protein segments . Most mutations lead to chain termination and result in secreted E-cadherin fragments. This explains why E-cadherin can no longer be detected in the tumor. The lack of E-cadherins due to carcinogenic mutation leads to ineffective binding among the tumor cells, as a result of which carcinoma cells become detached and can be carried by the bloodstream or lymph drainage into far, remote areas of the body where they can develop metastases. As tests on mice in which this gene was switched off, the absence of E-cadherin is incompatible with life. Embryonic development ends before the blastocyst stage. N-cadherin deficient mice die in the embryonic stage on day 9 or 10, as the differentiation of individual tissues such as e.g. B. of the heart does not take place.
Individual evidence
- ↑ a b c d e B. M. Gumbiner: Cell adhesion: Regulation of cadherin-mediated adhesion in morphogenesis. In: Nat Rev Mol Cell Biol . 2005.
- ↑ F. Nollet et al: Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. 2000.
- ↑ M. Takeichi: Cadherin cell adhesion receptors as a morphogeneity regulator. 1990.
- ↑ K. Hatta et al .: Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. 1987.
- ↑ M. Takeichi: The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. 1988.
- ↑ Derycke, Bracke, 2004.
- ↑ a b B. D. Angst et al .: The cadherin superfamily: diversity in form and function; Journal of Cell Science. 2001.
- ^ A b M. J. Wheelock, KR Johnson: Cadherins as modulators of cellular phenotype. In: Annual review of cell and developmental biology. 2003.
- ↑ K. Obst-Pernberg, C. Redies: Cadherins and synaptic specificity. In: Journal of neuroscience research. 1999.
- ↑ M. Frank, M. Ebert, W. Shan, GR Phillips, K. Arndt, DR Colman, R. Kemlera: Differential expression of individual gamma- protocadherins during mouse brain development. In: Mol. Cell. Neurosci. 29, 2005, pp. 603-616.
- ↑ Y. Li, DR Serwanski, CP Miralles, CG Fiondella, JJ Loturco, ME Rubio, AL De Blas: Synaptic and Nonsynaptic Localization of Protocadherin- gamma C5 in the Rat Brain. In: The Journal of Comparative Neurology. 518, 2010, pp. 3439-3463.
- ↑ D. Schreiner, JA Weiner: Combinatorial homophilic interaction between gamma- protocadherin multimers greatly expands the molecular diversity of cell adhesion. In: Proc Natl Acad Sci USA . 107, 2010, pp. 14893-14898.
- ^ S. Bonn, PH Seeburg, MK Schwarz: Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing. In: Mol Cell Biol. 27 (11), Jun 2007, pp. 4121-4132. Epub 2007 Apr 2.
- ↑ a b M. Conacci-Sorrel et al: The cadherin-catenin adhesion system in signaling and cancer. In: Journal of Clinical Investigation. 2002.
- ^ WJ Nelson, R. Nusse: Convergence of Wnt, {beta} -catenin, and cadherin pathways. In: Science. 2004.
- ↑ AR Brooks-Wilson et al .: Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. In: British Medical Journal. 2004.
- ↑ C. Redies: Cadherins in the central nervous system; Progress in neurobiology. 2000.