Osteolytic bone metastasis
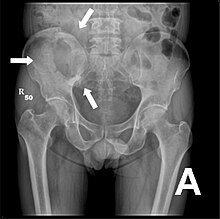
Osteolytic bone metastases are the most common bone metastases with a share of around 75% . Bone metastases arise almost exclusively in the medullary canal. The primary tumors are usually kidney, lung, breast or thyroid carcinomas. The strongly osteolytic multiple myeloma is not counted among the bone metastases in German-speaking countries.
Transforming growth factors ( transforming growth factor , TGF) such as TGF-β are stored in the bone matrix. The skeletal system is the largest reservoir for TGF-β. The tumor cells of the bone metastases secrete a the parathyroid hormone (PTH) related peptide, PTHrP ( parathyroid hormone-related protein ) having the same effect as parathyroid hormone and interleukin-11 (IL-11). Both proteohormones stimulate the osteoclasts, causing bone breakdown to proceed, as well as the release of growth factors that were originally immobilized in the bone matrix. These growth factors in turn stimulate the tumor cells to proliferate . This creates a vicious circle ( vicious circle ) of tumor progression and pathological bone remodeling, which is a major factor in the incurability of most bone metastases. Another factor that favors the growth of bone metastases is the lack of oxygen (hypoxia) in the area of the tumor cells.
The TGF-β signaling pathway is activated by the binding of TGF-β to the TGF type II receptor (TRII or TGF-IIR) and an approach to the TGF type I receptor (TRI or TGF-IR). The two receptors dimerize and activate a given in TRI kinase - domain to which the receptor-associated Smad2 and Smad3 (Smad = Mothers against decapentaplegic homolog ) attach. Smad2 and Smad3 are phosphorylated and together form the transcription factor Smad4 . Smad4 then transmits the TGF-β-mediated signal into the cell nucleus, where the target genes are activated. Via this signaling pathway, TGF-β not only stimulates the PTHrP and IL-11 genes, but also other genes that are important for colonization in bone. These include CTGF ( connective tissue growth factor ), interleukin-8 , CXCR4 (CXC motif chemokine receptor 4) and MMP1 (matrix metallopeptidase 1). In the mouse animal model , blocking the TGF-β signaling pathway reduced the number of bone metastases and increased survival, while overexpressing TRI reverses this effect. The same positive effects were observed when the TGF-β signal cascade was interrupted by gene knockout of Smad4 or by overexpression of Smad7 , a proteinogenic inhibitor, and various active ingredients with an inhibiting effect.
Individual evidence
- ↑ a b c S. Braun: Operative therapy and prognosis in patients with skeletal carcinoma metastases. Dissertation, Ludwig Maximilians University of Munich, 2004.
- ↑ GS Forbes, RA McLeod, RR Hattery: Radiographic manifestations of bone metastases from renal carcinoma. In: American Journal of Roentgenology Volume 129, Number 1, July 1977, pp. 61-66, ISSN 0361-803X . PMID 409145 .
- ↑ M. Oft, KH Heider, H. Beug: TGF beta signaling is necessary for carcinoma cell invasiveness and metastasis. In: Current biology Volume 8, Number 23, November 1998, pp. 1243-1252, ISSN 0960-9822 . PMID 9822576 .
- ^ DR Welch, A. Fabra, M. Nakajima: Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. In: Proceedings of the National Academy of Sciences Volume 87, Number 19, October 1990, pp. 7678-7682, ISSN 0027-8424 . PMID 2217201 . PMC 54811 (free full text).
- ^ A b G. R. Mundy: Metastasis to bone: causes, consequences and therapeutic opportunities. In: Nature Reviews Cancer Volume 2, Number 8, August 2002, pp. 584-593, ISSN 1474-175X . doi : 10.1038 / nrc867 . PMID 12154351 . (Review).
- ↑ a b c d L. K. Dunn, KS Mohammad, PG Fournier, CR McKenna, HW Davis, M. Niewolna, XH Peng, JM Chirgwin, TA Guise: Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. In: PloS One Volume 4, Number 9, 2009, p. E6896, ISSN 1932-6203 . doi : 10.1371 / journal.pone.0006896 . PMID 19727403 . PMC 2731927 (free full text). ( Open access )
- ↑ The term Mothers against decapentaplegic homolog is derived from Drosophila mothers against dpp (MAD; dpp = decapentaplegic protein) and is based on the name of charitable organizations such as Mothers Against Drunk Driving .
- ↑ K. Unsicker : Molecular Liberos. In: Ruperto Carola University of Heidelberg, Edition 3, 2001
- ^ J. Massagué, J. Seoane, D. Wotton: Smad transcription factors. In: Genes & development Volume 19, Number 23, December 2005, pp. 2783-2810, ISSN 0890-9369 . doi : 10.1101 / gad.1350705 . PMID 16322555 . (Review).
- ↑ Transforming growth factor β (TGF-β) In: DermoTopics Edition 1, 2002
- ↑ SM Kakonen, KS Selander, JM Chirgwin, JJ Yin, S. Burns, WA Rankin, BG Grubbs, M. Dallas, Y. Cui, TA Guise: Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. In: Journal of Biological Chemistry Volume 277, Number 27, July 2002, pp. 24571-24578, ISSN 0021-9258 . doi : 10.1074 / jbc.M202561200 . PMID 11964407 .
- ↑ MS Bendre, AG Margulies, B. Walser, NS Akel, S. Bhattacharrya, RA Skinner, F. Swain, V. Ramani, KS Mohammad, LL Wessner, A. Martinez, TA Guise, JM Chirgwin, D. Gaddy, LJ Suva: Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. In: Cancer Research Volume 65, Number 23, December 2005, pp. 11001-11009, ISSN 0008-5472 . doi : 10.1158 / 0008-5472.CAN-05-2630 . PMID 16322249 .
- Jump up ↑ Y. Kang, PM Siegel, W. Shu, M. Drobnjak, SM Kakonen, C. Cordón-Cardo, TA Guise, J. Massagué: A multigenic program mediating breast cancer metastasis to bone. In: Cancer Cell Volume 3, Number 6, June 2003, pp. 537-549, ISSN 1535-6108 . PMID 12842083 .
- ↑ JJ Yin, K. Selander, JM Chirgwin, M. Dallas, BG Grubbs, R. Wieser, J. Massagué, GR Mundy, TA Guise: TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. In: The Journal of clinical investigation Volume 103, Number 2, January 1999, pp. 197-206, ISSN 0021-9738 . doi : 10.1172 / JCI3523 . PMID 9916131 . PMC 407876 (free full text).
- ↑ M. Deckers, M. van Dinther, J. Buijs, I. Que, C. Löwik, G. van der Pluijm, P. ten Dijke: The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. In: Cancer research Volume 66, Number 4, February 2006, pp. 2202-2209, ISSN 0008-5472 . doi : 10.1158 / 0008-5472.CAN-05-3560 . PMID 16489022 .
- ^ Y. Kang, W. He, S. Tulley, GP Gupta, I. Serganova, CR Chen, K. Manova-Todorova, R. Blasberg, WL Gerald, J. Massagué: Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. In: Proceedings of the National Academy of Sciences Volume 102, Number 39, September 2005, pp. 13909-13914, ISSN 0027-8424 . doi : 10.1073 / pnas.0506517102 . PMID 16172383 . PMC 1236573 (free full text).
- ↑ D. Javelaud, KS Mohammad, CR McKenna, P. Fournier, F. Luciani, M. Niewolna, J. André, V. Delmas, L. Larue, TA Guise, A. Mauviel: Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. In: Cancer research Volume 67, Number 5, March 2007, pp. 2317-2324, ISSN 0008-5472 . doi : 10.1158 / 0008-5472.CAN-06-3950 . PMID 17332363 .
- ↑ R. Ge, V. Rajeev, P. Ray, E. Lattime, S. Rittling, S. Medicherla, A. Protter, A. Murphy, J. Chakravarty, S. Dugar, G. Schreiner, N. Barnard, M Reiss: Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. In: Clinical Cancer Research Volume 12, Number 14 Pt 1, July 2006, pp. 4315-4330, ISSN 1078-0432 . doi : 10.1158 / 1078-0432.CCR-06-0162 . PMID 16857807 .
- Jump up ↑ A. Bandyopadhyay, JK Agyin, L. Wang, Y. Tang, X. Lei, BM Story, JE Cornell, BH Pollock, GR Mundy, LZ Sun: Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I. receptor kinase inhibitor. In: Cancer research Volume 66, Number 13, July 2006, pp. 6714-6721, ISSN 0008-5472 . doi : 10.1158 / 0008-5472.CAN-05-3565 . PMID 16818646 .
- ↑ YA Yang, O. Dukhanina, B. Tang, M. Mamura, JJ Letterio, J. MacGregor, SC Patel, S. Khozin, ZY Liu, J. Green, MR Anver, G. Merlino, LM Wakefield: Lifetime exposure to A soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. In: The Journal of clinical investigation Volume 109, Number 12, June 2002, pp. 1607-1615, ISSN 0021-9738 . doi : 10.1172 / JCI15333 . PMID 12070308 . PMC 151015 (free full text).
- ↑ RS Muraoka, N. Dumont, CA Ritter, TC Dugger, DM Brantley, J. Chen, E. Easterly, LR Roebuck, S. Ryan, PJ Gotwals, V. Koteliansky, CL Arteaga: Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. In: The Journal of clinical investigation Volume 109, Number 12, June 2002, pp. 1551-1559, ISSN 0021-9738 . doi : 10.1172 / JCI15234 . PMID 12070302 . PMC 151012 (free full text).
- ↑ T. Hayashi, T. Hideshima, AN Nguyen, O. Munoz, K. Podar, M. Hamasaki, K. Ishitsuka, H. Yasui, P. Richardson, S. Chakravarty, A. Murphy, D. Chauhan, LS Higgins , KC Anderson: Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. In: Clinical cancer research Volume 10, Number 22, November 2004, pp. 7540-7546, ISSN 1078-0432 . doi : 10.1158 / 1078-0432.CCR-04-0632 . PMID 15569984 .
