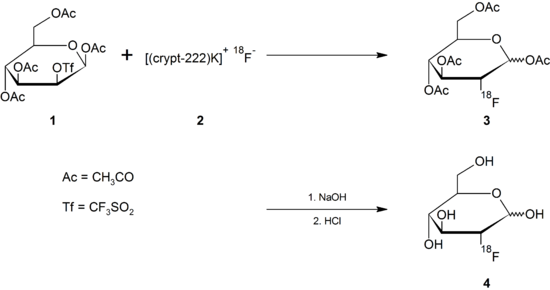Fluorodeoxyglucose
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Fludeoxyglucose ( 18 F) | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 11 FO 5 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
170-176 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2-fluoro-2-deoxy- D -glucose (simplified fluorodeoxyglucose , FDG for short ) is a structural analogue of the simple sugar D - glucose . Instead of an OH group, there is a fluorine atom at position 2 .
It is used as an in vivo diagnostic tool in medicine. The natural sugar and its mimetic FDG are initially absorbed by the cells of the human body in the same way, but are then metabolized differently. This results in an intended enrichment of the diagnostic agent in certain body cells. The concentration of the FDG tracer in the tissues to be examined is recorded tomographically . In this way, deviations from the norm are localized and indications of organic malfunctions are obtained. FDG labeled with the radionuclide fluorine-18 ( 18 F) is the most frequently used radiopharmaceutical in positron emission tomography . Radiation-free FDG is experimentally tested in magnetic resonance tomography . FDG is not approved as a therapeutic agent .
history
The synthesis of fluorodeoxyglucose was first developed in 1968 by Josef Pacák, Zdeněk Točík and Miloslav Černý at Charles University in Prague . In 1970 an alternative synthetic route was published. The property of FDG to inhibit the enzyme hexokinase , which is important for the sugar metabolism , was described in 1972. Tatsuo Ido, Alfred P. Wolf and Joanna Fowler from Brookhaven National Laboratory were the first to describe the synthesis of radioactive 18 F-FDG in 1976 . The first synthesis Tatsuo Ido succeeded in the group of Wolf. This compound was injected by Abass Alavi into two volunteers at the University of Pennsylvania in August that year . The first images of the brain, in which the distribution of FDG in this organ was shown for the first time, were taken with a conventional gamma camera , not with a positron emission tomograph. In 1984 a process was described which, after a further development in 1986, has served as a template for the manufacture of the radiopharmaceutical since then. Today it is used in oncology and neurology, where it is by far the most widely used diagnostic tool.
synthesis
The short half-life of the radioactive fluorine isotope places high demands on the production of the radiopharmaceutical. Rapid synthesis, cleaning and sterilization processes must be ensured. Today, the production of 18 F-2-fluoro-2-deoxy- D -glucose is automated. The scope of quality control is limited by the circumstances.
A number of possible reactions for the production are described. There are mainly two synthetic routes: the electrophilic addition , i.e. the addition of 18 F-F 2 to double bonds, and the nucleophilic substitution with 18 F - .
Nucleophilic substitution
The route via nucleophilic substitution is the routine route and is described here by way of example, with the protective groups being removed either with acids or bases in the various processes of nucleophilic substitution .
Making 18 F -
In this case, the natural fluorine atom with nucleon number 19 in the 2-FDG molecule has been replaced by the radioactive fluorine-18 isotope . This isotope is a positron emitter with a half-life of only 109.8 minutes. It doesn't exist in nature because of its rapid decay . To produce 18 F-2-FDG, this isotope is usually obtained with the help of a cyclotron , for example by bombarding the heavy oxygen isotope 18 O with protons or from 20 Ne . Since direct irradiation of normal glucose with high-energy protons for the intended formation of 18 F-2-FDG only leads to the destruction of the organic molecule, the radioactive isotope must be produced separately with the help of a cyclotron and then processed further.
In a cyclotron, a target made of water H 2 18 O enriched with the oxygen isotope 18 O is bombarded with high-energy protons (approx. 15 MeV ). In a nuclear reaction, a small part of the 18 O oxygen is converted into the radioactive fluorine isotope 18 F by taking up a proton and releasing a neutron .
Implementation with a precursor
The fluoride formed is separated from the water via an ion exchanger and then separated (eluted) from the ion exchanger with a solution of acetonitrile , Kryptofix 222 and potassium carbonate ( 2 ).
In the actual nucleophilic substitution, the radioactive fluoride ion replaces an easily removable leaving group , such as a triflate . A suitable precursor for the production of 2-FDG by means of nucleophilic substitution is 1,3,4,6- O- acetyl-2- O -trifluoromethanesulfonyl-β- D -mannopyranose ( 1 ), referred to as mannose triflate for short . After the substitution of the triflate, the four acetyl protective groups are removed by means of basic or acidic hydrolysis with dilute sodium hydroxide solution or dilute hydrochloric acid ( 4 ).
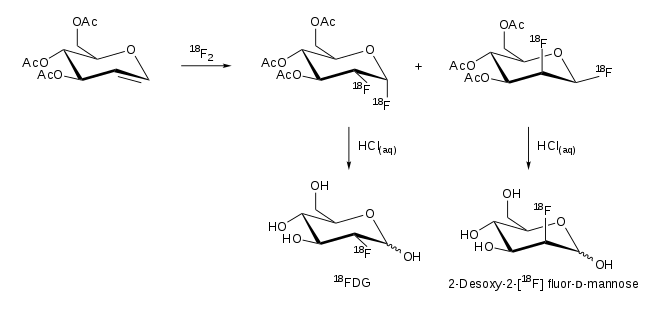
Electrophilic addition
In addition, there is the synthesis route of addition with 18 F-F 2 . A reaction of 3,4,5-tri- O -acetyl- D -glucal produces two products, 2-FDG and 2-fluorodeoxymannose:
Purification
The 2-FDG formed can be separated cleanly from the starting materials by solid phase extraction or reverse phase high performance liquid chromatography (RP-HPLC). Purification is an important step in the production of 2-FDG in order to meet the requirements of the individual pharmacopoeias and Good Manufacturing Practice (GMP). According to the European Pharmacopoeia , more than 95% of the radioactivity must come from fluorodeoxyglucose and fluorodeoxymannose , whereby the proportion of fluorodeoxymannose may not exceed 10% of the radioactivity. The purification steps take place in closed devices shielded with lead cells.
metabolism
18 F-fluorodeoxyglucose is absorbed by the cells of the human body like glucose, although a hydroxyl group has been replaced by the radionuclide 18 F at one point on the molecule . In this process, 2-FDG is taken up by the cells from the blood by means of glucose transporters . As part of the human genome project, 14 glucose transporters (GLUT) were identified in humans.
GLUT-1 is the most important transport protein for the uptake of 2-FDG in tumors and normal brain tissue. The uptake in the skeletal muscles and the heart muscle can be stimulated by insulin and takes place via the GLUT-4 transporter. The enzyme hexokinase then phosphorylates 2-FDG within the cell. However, 2-FDG cannot be further metabolized by the cells after phosphorylation. The reverse reaction , the dephosphorylation of FDG-6 phosphate to FDG, takes place very slowly in all organs - with the exception of the liver - and in tumor tissue. Therefore, FDG-6 phosphate accumulates in the cells ( metabolic trapping ). Based on the decay of 18 F, 2-FDG can be detected. The distribution of 2-FDG in the body allows conclusions to be drawn about the glucose metabolism of various tissues. This is of particular advantage for the early diagnosis of cancer, since a tumor cell typically consumes a lot of glucose due to an increased metabolism and accordingly accumulates 2-FDG. The metabolic activity of a tumor is described quantitatively with the help of the SUV value.
As a molecule very similar to glucose, 2-FDG easily crosses the blood-brain barrier . Since the human brain has a high requirement for glucose, a correspondingly large proportion of 2-FDG is accumulated in the brain. In addition, in healthy people, 2-FDG accumulates in the kidneys and in the urinary tract.
The 18 F breaks down to 18 O, a natural isotope of oxygen. Immediately after the decomposition, a hydroxyl group is formed after a free hydrogen atom has been taken up from the environment . Thus, after radioactive decay, “normal” glucose is created from the 2-FDG.
The glucose formed is then metabolized in the normal way in glycolysis , while 18 F-2-FDG phosphate inhibits the enzyme in the subsequent glycolysis reaction step ( glucose-6-phosphate isomerase ). Tumor cells also do not have sufficient amounts of the enzyme glucose-6-phosphatase to catalyze the reverse reaction of 18 F-2-FDG-phosphate to 18 F-2-FDG.
The imaging possible through the breakdown of 18 F-2-FDG is an image of the distribution of glucose uptake and phosphorylation of cells in the human body. FDG also shows increased uptake in tumors of mice, rats, hamsters and rabbits. In rats, 2-FDG in tumors, due to the increased metabolic activity, shows an accumulation 22-fold compared to the blood circulation one hour after injection, which remains constant for a further hour.
Applications
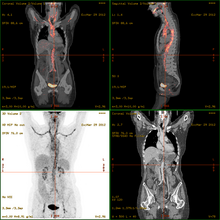
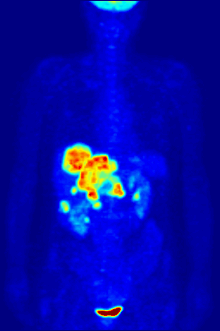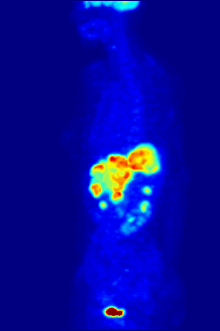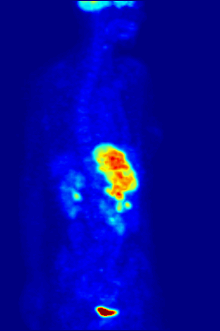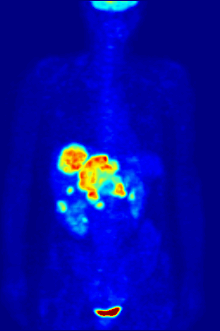
2-FDG is used in PET for diagnosis , staging , therapy adjustment and therapy control. In this context one often speaks of the "FDG-PET". As a diagnostic agent, 2-FDG is an extremely useful and proven compound. The application has a purely diagnostic background. The gamma radiation produced by the annihilation of the positron and electron is used . Annihilation produces two high-energy photons (gamma quanta) that have an energy of 511 keV and are emitted at an angle of 180 degrees to each other. The gamma quanta used for diagnostics are not suitable for therapy ( radiation therapy , in this particular case one would speak of endoradiotherapy).
In addition to its main application in oncology, 2-FDG is also used for the diagnosis of Alzheimer's disease , Parkinson's disease , epilepsy , in cardiology and in inflammation diagnostics , albeit to a far lesser extent than in oncology.
For the FDG-PET there are three main indications for the examination of patients with oncological diseases: the differentiation between benign or malignant (benign or malignant) tumors, the tumor staging with regard to the lymph nodes and distant metastases as well as the differentiation between scar tissue and vital tumor tissue ( recurrence , residual Tumor). The FDG-PET is used in oncology to examine lung cancer , colorectal cancer , esophageal cancer , stomach cancer , head and neck cancer , cervical cancer , ovarian cancer , breast cancer , malignant melanoma and most types of lymphoma . In particular, very slowly growing tumors generally do not show a significantly increased FDG uptake. An FDG-PET examination is usually only useful in exceptional cases. These include prostate carcinomas , differentiated neuroendocrine tumors (e.g. carcinoid ), bronchoalveolar carcinomas, low-grade non-Hodgkin lymphomas , low-grade brain tumors ( astrocytoma II, oligodendroglioma II) and hepatocellular carcinoma (especially more highly differentiated forms). In addition to the tumor tissue, inflammations or healings also show increased metabolic activity and thus increased FDG uptake. An examination to differentiate, for example, abscesses and tumor tissue, sarcoidosis , bronchial carcinoma , etc., can therefore hardly be carried out meaningfully with 2-FDG.
High blood sugar levels lead to a reduced uptake of 2-FDG in tumor tissue, which worsens the signal-to-noise ratio . In the case of fasting blood sugar levels above 150 mg / dl, the indication for FDG-PET is therefore usually checked critically. After completion of chemotherapy or radiation therapy, there is often a reduction in FDG uptake even with vital tumor cells. Therefore, there should be a period of at least four weeks between the PET examination and the completion of therapy. Follow-up examinations and certain clinical studies are an exception . Alternatives to 2-FDG are e.g. B. radiolabeled amino acids such as 11 C- methionine for determining the protein biosynthesis , radiolabeled 11 C - choline for determining the synthesis of membrane lipids and radiolabeled 11 C- acetate for the determination of fatty acid synthesis . However, only 2-FDG is approved as a tracer by the Food and Drug Administration in the USA . In Germany, FDG was approved as a tracer in 2000 and 2005.
application
In the case of the whole-body scan looking for tumors or their metastases , a dose of around 200 to 400 MBq is injected into a vein of the patient using an isotonic saline solution . The amount of activity to be applied is calculated from the patient's body surface . The goal is to be able to register around 210,000 events per shift in the PET. The maximum recommended dose of FDG solution for injection is 10 mg, equivalent to 55 micromoles .
Before applying 2-FDG, the patient must fast for at least six hours in order to have the lowest possible blood sugar level . This requirement is problematic for some diabetics , as the corresponding clinics usually do not carry out a PET examination if the blood sugar level is above 10 mmol / l. The venous blood sugar level is determined before each FDG-PET examination.
After the injection, the patient usually has to lie as completely as possible for an hour without physical activity in order to ensure the even distribution of 2-FDG in the body. Muscular exertion would direct 2-FDG to the corresponding muscles and falsify the result or lead to artifacts in the imaging. A strong accumulation of 2-FDG is often observed in the area of the tongue, which is caused by frequent and strong swallowing movements of the patients, some of whom are under extreme psychological stress .
The patient can drink water or other calorie-free beverages prior to application. Immediately before starting the exposure, the patient should empty the bladder.
Radiation exposure
The radiation dose for PET with 2-FDG is approx. 7-10 mSv . In comparison, the radiation dose for contrast-enhanced computed tomography is approx. 20–40 mSv. The level of that dose corresponds to about double to triple the dose of natural radiation exposure to which the European population is exposed annually (approx. 3 mSv per year). For this reason, the risk of side effects from radiation occurring is negligibly small. The effective dose after intravenous injection of 2-FDG is 2.0 × 10 −2 mSv / MBq. The highest radiation exposure for the urinary bladder is 1.7 × 10 −1 mSv / MBq. After an application, breastfeeding should not take place for at least 10 half-lives (1098 minutes, corresponding to about 18 hours) .
Side effects
There are no known allergic or toxic side effects. The extremely low dose of injected 2-FDG in the range picomoles to nanomoles is located, and the comparatively mild type of radiation exclude this. In comparison , the amounts of contrast media used in CT or magnetic resonance tomography are in the range of a few millimoles, that is, they are approximately 6 to 9 orders of magnitude higher.
Therapeutic uses
While the gamma radiation from 2-FDG is largely useless therapeutically, the β + radiation of the positron, similar to the β - radiation of an electron, has therapeutic potential. This is mainly due to the short range of the positrons in the tumor tissue - it is only 1 to 2 mm - and the rather high selective accumulation of 2-FDG in the tumor tissue. Initial studies have been carried out in melanoma , breast cancer and colorectal cancer . An approval for the treatment of cancer has not been 2-FDG.
literature
- Klaus Wienhard, Rainer Wagner, Wolf-D. Hot: PET. Basics and applications of positron emission tomography. Springer, Berlin et al. 1989, ISBN 3-540-19451-7 .
- Klaus Kopka, Otmar Schober , Stefan Wagner: 18 F-labeled cardiac PET tracers: selected probes for the molecular imaging of transporters, receptors and proteases , in: Basic Research in Cardiology , 2008, 103 (2), pp. 131-143; doi: 10.1007 / s00395-008-0703-6 ; PMID 18324369 .
- A. Zhu, DM Marcus, HK Shu, H. Shim: Application of Metabolic PET Imaging in Radiation Oncology , in: Radiation Research , 2012, 177 (4), pp. 436-448; PMID 22339451 ; PMC 3922713 (free full text, PDF).
Web links
Individual evidence
- ^ A b Josef Pacák, Zdeněk Točík, Miloslav Černý: Synthesis of 2-Deoxy-2-fluoro- D -glucose , in: Journal of the Chemical Society D: Chemical Communications , 1969, pp. 77-77; doi: 10.1039 / C29690000077 .
- ↑ a b Data sheet 2-Fluoro-2-deoxy- D -glucose from Sigma-Aldrich , accessed on November 16, 2016 ( PDF ).
- ^ J. Adamson, AB Foster, LD Hall, RN Johnson, RH Heese: Fluorinated carbohydrates: Part III. 2-deoxy-2-fluoro- D -glucose and 2-deoxy-2-fluoro- D -mannose , in: Carbohydrate Research , 1970, 15 (3), pp. 351-359; doi: 10.1016 / S0008-6215 (00) 80451-6 .
- ^ EM Bessell, AB Foster, JH Westwood: The Use of Deoxyfluoro- D -glucopyranoses and Related Compounds in a Study of Yeast Hexokinase Specificity , in: The Biochemical Journal , 1972, 128 (2), pp. 199-204, PMID 4563639 , PMC 1173755 (free full text, PDF).
- ↑ a b T. Ido, CN Wan, JS Fowler , AP Wolf: Fluorination with F 2 . A Convenient Synthesis of 2-Deoxy-2-fluoro- D -glucose , in: J. Org. Chem. , 1977, 42 (13), pp. 2341-2342; doi: 10.1021 / jo00433a037 .
- ↑ a b T. Ido, C.-N. Wan, V. Casella, JS Fowler, AP Wolf, M. Reivich, DE Kuhl: Labeled 2-deoxy-D-glucose analogs: 18 F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2 -fluoro-D-mannose and 14 C-2-deoxy-2-fluoro-D-glucose , in: Journal of Labeled Compounds and Radiopharmaceuticals , 1978, 14 (2), pp. 174-183; doi: 10.1002 / jlcr.2580140204 .
- ↑ Joanna S. Fowler, Michael J. Welch: Alfred P. Wolf, Biographical Memoirs National Academy of Sciences, Volume 78, p. 361, online
- ↑ Abass Alavi, Martin Reivich: The Conception of FDG-PET Imaging , in: Seminars in Nuclear Medicine , 2002, 32 (1), pp. 2-5; doi: 10.1053 / snuc.2002.29269 ; PMID 11839067 .
- ↑ K. Hamacher: Phase-transfer catalysed synthesis of 4-S-β- D -glucopyranosyl-4-thio- D -glucopyranose (thiocellobiose) and 2-S-β- D -glucopyranosyl-2-thio- D -glucopyranose ( thiosophorose) , in: Carbohydrate Research , 1984, 128 (2), pp. 291-295; doi: 10.1016 / 0008-6215 (84) 85336-7 .
- ↑ K. Hamacher, HH Coenen, G. Stöcklin: Efficient Stereospecific Synthesis of No-Carrier-Added 2- [ 18 F] -Fluoro-2-Deoxy- D -glucose Using Aminopolyether Supported Nucleophilic Substitution , in: Journal of Nuclear Medicine , 1986, 27 (2), pp. 235-238; PMID 3712040 ; PDF .
- ^ Gesellschaft Deutscher Chemiker: Annexes to the position paper of the Nuclear Chemistry Section ( Memento from March 31, 2010 in the Internet Archive ), February 2000.
- ↑ Simone Maschauer, Olaf Prante: Sweetening Pharmaceutical Radiochemistry by 18 F-Fluoroglycosylation: A Short Review , in: BioMed Research International , Volume 2014, Article ID 214748; doi: 10.1155 / 2014/214748 ; PMID 24991541 ; PMC 4058687 (free full text, PDF).
- ↑ a b S. Yu: Review of 18 F-FDG synthesis and quality control , in: Biomedical Imaging and Intervention Journal , 2006, 2 (4), e57; PMID 21614337 ; PMC 3097819 (free full text, PDF).
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties , in: Nuclear Physics A , 729, 2003, pp. 3–128, here: p. 29. doi : 10.1016 /j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ a b c d e Harald Schicha: Nuclear Medicine. Schattauer Verlag, 2007, ISBN 978-3-794-52438-9 , pp. 55-58.
- ^ R. Lambrecht, AP Wolf: Cyclotron and short-lived halogen isotopes for radiopharmaceutical applications. In: Radiopharmaceuticals and Labeled Compounds , International Atomic Energy Agency, Vienna 1973, m. 1: pp. 275-290.
- ↑ Ali Mobasheri: Facilitative glucose transporter in Articular Chondrocytes. Springer Science & Business Media, 2008, ISBN 978-3-540-78899-7 , p. 22 ( limited preview in Google book search).
- ↑ a b c d e f g h i j TU Munich: Positron Emission Tomography (PET) for oncological issues ( Memento from September 29, 2007 in the Internet Archive ).
- ^ Daniel N. DeMaio: Mosby's Exam Review for Computed Tomography. Elsevier Health Sciences, 2010, ISBN 978-0-323-06589-4 , p. 184.
- ↑ a b Kam Leung: [ 18 F] Fluoro-2-deoxy-2- D -glucose , in: Molecular Imaging and Contrast Agent Database (MICAD) , 2005; Full text . PMID 20641537 .
- ↑ P. Som, HL Atkins, D. Bandoypadhyay, JS Fowler, RR MacGregor, K. Matsui, ZH Oster, DF Sacker, CY Shiue, H. Turner, CN Wan, AP Wolf, SV Zabinski: A Fluorinated Glucose Analog, 2 -fluoro-2-deoxy- D- glucose (F-18): Nontoxic Tracer for Rapid Tumor Detection , in: Journal of Nuclear Medicine , 1980, 21 (7), pp. 670-675, PMID 7391842 ; PDF .
- ↑ H. Fukuda, T. Matsuzawa, Y. Abe, S. Endo, K. Yamada, K. Kubota, J. Hatazawa, T. Sato, M. Ito, T. Takahashi, R. Iwata, T. Ido: Experimental Study for Cancer Diagnosis with Positron-Labeled Fluorinated Glucose Analogs: [ 18 F] -2-Fluoro-2-Deoxy-D-Mannose: A new Tracer for Cancer Detection , in: European Journal of Nuclear Medicine , 1982, 7 (7) , Pp. 294-297; doi: 10.1007 / BF00253423 ; PMID 6981508 .
- ↑ E. Croteau, JM Renaud, MA Richard, TD Ruddy, F. Bénard, RA deKemp: PET Metabolic Biomarkers for Cancer , in: Biomarkers in Cancer , 2016, 8 , Suppl 2, pp. 61-69; doi: 10.4137 / BIC.S27483 ; PMID 27679534 ; PMC 5030827 (free full text, PDF).
- ^ S. Hofman, RJ Hicks: How We Read Oncologic FDG PET / CT , in: Cancer Imaging : the official publication of the International Cancer Imaging Society , 2016, 16 (1), p. 35; doi: 10.1186 / s40644-016-0091-3 ; PMID 27756360 ; PMC 5067887 (free full text, PDF).
- ↑ S. Segobin, R. La Joie, L. Ritz, H. Beaunieux, B. Desgranges, G. Chételat, AL Pitel, F. Eustache: FDG-PET Contributions to the Pathophysiology of Memory Impairment , in: Neuropsychology Review , 2015 , 25 (3), pp. 326-355; doi: 10.1007 / s11065-015-9297-6 ; PMID 26319237 .
- ^ I. Sarikaya: PET imaging in neurology: Alzheimer's and Parkinson's diseases , in: Nuclear Medicine Communications , 2015, 36 (8), pp. 775-781; doi: 10.1097 / MNM.0000000000000320 ; PMID 25920047 .
- ↑ JG Burneo, R. Poon, S. Kellett, OC Snead: The Utility of Positron Emission Tomography in Epilepsy , in: Canadian Journal of Neurological Sciences . Le journal canadien des sciences neurologiques , 2015, 42 (6), pp. 360–371; doi: 10.1017 / cjn.2015.279 ; PMID 26437611 .
- ↑ D. Jain, ZX He, V. Lele: Cardiac Hot Spot Imaging With 18 FDG , in: Seminars in Nuclear Medicine , 2014, 44 (5), pp. 375-385; doi: 10.1053 / j.semnuclmed.2014.06.010 ; PMID 25234081 .
- ^ S. Vaidyanathan, CN Patel, AF Scarsbrook, FU Chowdhury: FDG PET / CT in infection and inflammation-current and emerging clinical applications , in: Clinical Radiology , 2015, 70 (7), pp. 787-800; doi: 10.1016 / j.crad.2015.03.010 ; PMID 25917543 .
- ↑ a b c A. Zhu, DM Marcus, HK Shu, H. Shim: Application of Metabolic PET Imaging in Radiation Oncology , in: Radiation Research , 2012, 177 (4), pp. 436-448; PMID 22339451 ; PMC 3922713 (free full text, PDF).
- ↑ K.-J. Langen, U. Braun, E. Rota Kops, H. Herzog, T. Kuwert, B. Nebeling, LE Feinendegen: The Influence of Plasma Glucose Levels on Fluorine-18-Fluorodeoxyglucose Uptake in Bronchial Carcinomas , in: Journal of Nuclear Medicine , 1993, 34 , pp. 355-359; PMID 8441023 ; PDF .
- ↑ a b Clinical application of FDG-PET Scan ( Memento of July 3, 2007 in the Internet Archive ).
- ↑ How high is the radiation exposure? ( Memento of September 30, 2007 in the Internet Archive ).
- ^ Radiation Dose to Patients from Radiopharmaceuticals , ICRP Publication 53, Pergamon Press, 1987.
- ^ Recommendations of the International Commission on Radiological Protection , ICRP Publication 60, Pergamon Press, 1990.
- ^ Lennart Johansson, Sören Mattsson, Bertil Nosslin, Sigrid Leide-Svegborn: Effective dose from radiopharmaceuticals , in: European Journal of Nuclear Medicine , 1992, 19 , pp. 933-938; doi: 10.1007 / BF00175858 ; PMID 1308762 .
- ↑ Jerome Z. Litt: Litt's Drug Eruptions & Reactions Manual, 17th Edition. CRC Press, 2011, ISBN 978-1-841-84837-2 , p. 268.
- ↑ Renee M. Moadel, Andrew V. Nguyen, Elaine Y. Lin, Ping Lu, Joseph Mani, M. Donald Blaufox, Jeffrey W. Pollard, Ekaterina Dadachova: Positron emission tomography agent 2-deoxy-2- [ 18 F] fluoro - D- glucose has a therapeutic potential in breast cancer , in: Breast Cancer Research , 2003, 5 (6), pp. R199-R205; doi: 10.1186 / bcr643 ; PMID 14580255 ; PMC 314404 (free full text, PDF).
- ↑ S. Jaini, E. Dadachova: FDG for therapy of metabolically active tumors , in: Seminars in Nuclear Medicine , 2012, 42 (3), pp. 185-189; doi: 10.1053 / j.semnuclmed.2011.12.001 ; PMID 22475427 (Review).