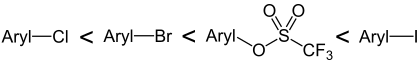Triflyl group
The triflyl group (CF 3 SO 2 - ) is the covalently bonded residue of trifluoromethanesulfonic acid . These are mostly the esters of trifluoromethanesulfonic acid. Their name is derived from its systematic name Trifl uormethansulfon yl off. The compounds are usually referred to as triflates and should not be confused with the salts of the same name of trifluoromethanesulfonic acid ( triflates ). In structural formulas, the triflyl group is often abbreviated as Tf; it is structurally related to the mesyl group . The triflate group is often abbreviated to OTf and is similar to the mesylate group .
Manufacturing
Triflates can be produced by deprotonating an alcohol and then reacting with trifluoromethanesulfonyl chloride, whereby one equivalent of hydrogen chloride is bound as the hydrochloride by a base :
properties
Due to the negative inductive effects of the fluorine atoms, the sulfur and oxygen atoms are very poor in electrons. In addition, the triflation is well mesomeric stabilized over three boundary structures . This leads to the fact that the trifluoromethanesulfonic acid is a strong acid and thus the triflation is a weak base , which can easily be split off with substitution by suitable nucleophiles . For this reason, triflyl compounds must be stored in the absence of nucleophiles, for example water or alcohols.
use
The triflylate group is an excellent leaving group and as such is used in preparative organic chemistry . By converting alcohols into triflates, the poor leaving group OH - is converted into a good leaving group (CF 3 SO 3 - ), which enables substitution reactions at this position. Triflates are up to 10,000 times more reactive than tosylates .
This application is not particularly atom-economical , since stoichiometric amounts of waste materials with a comparatively high molecular mass are always formed. This is why the synthesis method is mainly used on a laboratory scale and rarely in technical processes.
With alcohols , alkyl triflates form ethers in the sense of a Williamson ether synthesis .
In palladium- catalyzed reactions such as the Suzuki or Sonogashira coupling , aryl or alkenyl triflates can be used instead of the halogen compounds that are frequently used. The series of reactivity of the aryl compounds is:
11 C-labeled methyl triflate is used to introduce labeled methyl groups into biological molecules.
Individual evidence
- ^ Author collective, Organikum , 21st edition, p. 217, Wiley-VCH, Weinheim, 2001, ISBN 3-527-29985-8 .
- ↑ a b Entry on trifluoromethanesulfonic acid. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ^ Author collective, Organikum , 21st edition, pp. 405-407, Wiley-VCH, Weinheim, 2001, ISBN 3-527-29985-8 .