Mannose
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
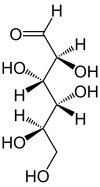
| Fischer projection , open-chain representation | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Mannose | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O 6 | |||||||||||||||
| Brief description |
colorless and odorless, crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 180.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.54 g cm −3 ( D- mannose) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mannose , often abbreviated as Man in biochemical terms , is an epimer of glucose , more precisely a C2 epimer. As D- mannose , it is a natural hexose and a component of numerous plant polysaccharides (mannans). In the organism it is mainly part of membranes. In relation to sucrose , a 10% solution has a sweetness of 59%.
The name is derived from mannitol , more details on the etymology can be found in the corresponding article.
Whenever "mannose" is mentioned in this text or in the scientific literature without any additional name ( prefix ), it means D- mannose. The (unnatural) L- mannose is synthetically accessible and is of only minor importance.
properties
Behavior in aqueous solution
In aqueous solution, an intramolecular ring closure sometimes occurs, so that an equilibrium is established between the aldehyde form and the two ring forms ( furanose form and pyranose form), with mannose then being almost exclusively in the pyranose form:
| D -Mannose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |
|
 α- D -mannofuranose <1% |
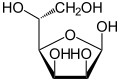 β- D -mannofuranose <1% |
 α- D -mannopyranose 67% |
 β- D- mannopyranose 33% |
|
Mannose synthesis
By the glucose-6-phosphate isomerase is glucose-6-phosphate in analogy to glycolysis in fructose-6-phosphate is converted, which in turn by means of the mannose-6-phosphate isomerase to mannose-6-phosphate isomerized is. Another rearrangement to mannose-1-phosphate is catalyzed by the enzyme phosphomannomutase . Mannose is industrially produced from woods such as birch and beech, but also from corn.
Degradation of mannose
If free mannose gets into a cell, it is phosphorylated to mannose-6-phosphate by means of the enzyme hexokinase , which means that it can no longer leave the cell because there is no suitable transport protein for it in the cell membrane. When it is not needed to build up new glycoproteins , it is converted to fructose-6-phosphate via mannose-phosphate isomerase, which in turn can be fed to glycolysis with energy gain.
GDP mannose
A special feature of mannose is that it is activated not via uridine triphosphate (UTP) but via guanosine triphosphate (GTP). For this, mannose-6-phosphate is first converted to mannose-1-phosphate, which then reacts further to form GDP-mannose. The reaction sequence is the same as in the reaction of glucose to UDP-glucose and in glycogen synthesis .
Insect toxicity
While mannose is almost non-toxic to humans, it is highly toxic to various hymenoptera such as bees (which also includes bumblebees ) and the common wasp Vespa vulgaris . A lethal dose of 0.4–0.5 mg was determined in bees . The poisonous effect is based on the similarity of mannose to glucose , which leads to a competitive inhibition of different enzymes .
Use in medicine
According to two studies, D- Mannose can be used therapeutically for the prophylaxis of cystitis . However, other publications question this statement.
Web links
Individual evidence
- ↑ a b c d L-Mannose data sheet at Acros, accessed March 23, 2007.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 , pp. 282 ( limited preview in Google Book search).
- ↑ a b c Entry on mannose. In: Römpp Online . Georg Thieme Verlag, accessed on October 12, 2011.
- ↑ a b D-Mannose data sheet (PDF) from Calbiochem, accessed on December 7, 2015.
- ^ Hans-Dieter Belitz, Werner Grosch, Peter Schieberle: Textbook of food chemistry . 6th, completely revised edition Springer, Berlin 2008, ISBN 978-3-540-73201-3 , p. 263.
- ↑ Helge May: Hummelsterben im Nektarloch. In: Archive Nature Conservation Today. Retrieved on September 20, 2014 (originally from: Naturschutz heute. Issue 3, 1995).
- ↑ Theodor Staudenmayer: The toxicity of mannose for bees and other insects. In: Journal of Comparative Physiology . Vol. 26, No. 5, 1939, pp. 644-668, doi: 10.1007 / BF00341096 .
- ↑ Information on the scientific studies. [1] Kranjcec et al. a. (2014) and [2] Porru u. a. (2014). On Medizin-transparent.at, accessed on February 11, 2019.
- ↑ Urinary tract infections: Sugar protects as well as antibiotics. In: ÄrzteZeitung of August 16, 2013. On Aerztezeitung.de, accessed on February 11, 2019.
- ↑ With mannose against cystitis? November 24, 2017. From Medizin-transparent.at, accessed on February 11, 2019. However, see the discussion page.