Electrophilic addition
The electrophilic addition (A E ) is a chemical reaction in organic chemistry in which unsaturated hydrocarbons ( alkenes or alkynes ) react with different classes of substances. A common feature of electrophilic additions is that the reaction is initiated by the attack of an electron-loving particle, the electrophile , on the double or triple bond, more precisely on the π bond.
Addition to the C = C double bond
Addition of halogens to alkenes
Molecular halogens can be added to the double bond of alkenes in a two-step mechanism . The halogen molecule interacts with the double bond of the alkene, as a result of which the halogen molecule is polarized and the electron bond is split heterolytically . In the first step, a halonium ion (here as an example: a bromonium ion (Br + )) is added to the double bond of the carbon atom , in which it forms a short-lived cyclic cation while splitting the double bond . In the second step, the halide ion (here: bromide ion ) attacks nucleophilically on the positively charged carbon atom, whereby the saturated α, β-dihalogenated product is obtained.
The addition of halogens is limited to the elements chlorine , bromine and iodine . Molecular fluorine, on the other hand, is highly reactive and would attack CC and CH bonds unselectively. Chlorine is more electronegative and less polarizable than bromine and iodine. For this reason, the addition of chlorine takes place via a carbocationic intermediate stage, which is not stabilized by the formation of a cyclic (non-existent) chloronium structure.
The formation of stabilized cations, as occurs for the addition of bromine and iodine, also determines the stereochemistry of the dihalide formed. This effectively shields one side of the molecule from nucleophilic attacks, so that the attack can only take place from the opposite side. One speaks here of an anti- addition. As a result, the addition of bromine and iodine results in the anti product being formed with high selectivity .
Addition of hydrogen halides
Also hydrogen halides can be added to alkenes, which haloalkanes are formed. This addition also takes place in two stages. In the first step, the proton of the acid used adds to the double bond. In contrast to halonium ions, the proton does not have the ability to stabilize the positive charge, which is why a carbocation is formed. In the second step, the anion of the acid is added to this .
With this addition, two different products can be formed which differ in the position of the halogen. Which of the products is preferentially formed depends on the stabilization of the intermediate carbocation and is described by Markovnikov's rule , which states that the hydrogen atom is always bound to the carbon atom that is already richer in hydrogen. The product is preferably formed which has a better stabilized carbocation. As a rule, the more stable carbocation is the more highly alkylated . Depending on the starting material , high regioselectivities can be achieved in this reaction .
Alkenes Alcohols
Addition of water to alkenes
Water is a bad nucleophile , which is why the reaction of alkenes with water usually does not lead to the expected reaction product, an alcohol . However, the reaction takes place under acid catalysis. As previously described, a proton of the acid is added to the double bond in the first step. The formed carbocation is now sufficiently electrophilic for the nucleophilic attack of a water molecule. This splits off a proton after the addition, creating a secondary alcohol.
If the anion of the acid is itself a nucleophile, the anion competes with water for addition to the carbocation (see also previous section).
Synthesis of diols
The addition of water to double bonds gives alcohols, but diols cannot be synthesized in this way . These are possible through the addition of inorganic oxygen compounds, for example potassium permanganate or osmium tetroxide . In the first step, the oxygen carrier adds to the alkene, and in the second step the intermediate product is hydrolyzed, which releases the diol.
In 2001 , Barry Sharpless was awarded the Nobel Prize in Chemistry for the development of a stereoselective dihydroxylation .
→ see also main article: Dihydroxylation
Addition to a conjugated double bond
The addition to conjugated double bonds follows the same rules as the addition to isolated double bonds described above. However, it must be taken into account that the carbocation formed in the first step can be stabilized mesomerically . This stabilization distributes the positive charge to several carbon atoms, which means that the nucleophile can attack at different positions. Usually a mixture of the two products is obtained in such reactions.
Addition to alkynes
Analogously to the addition to alkenes, additions to the triple bond of alkynes can also be carried out. Mechanistically, these proceed analogously to the addition to alkenes. In the first step, an electrophile attacks the triple bond, creating a vinyl cation. In the second step, an existing nucleophile then attacks the cationic position. This creates a substituted alkene. This can react further in a second addition reaction to form the corresponding alkane .
If electron-withdrawing groups such as halogen atoms were introduced by the first addition, the second addition proceeds significantly more slowly because the electron density of the double bond is reduced. The reaction can often be stopped after the first addition at the alkene stage.

The acid-catalyzed addition of water to alkynes yields enols . These tautomerize to ketones ( keto-enol tautomerism ), which is why the addition of water to alkynes is a possibility for the synthesis of ketones.
Addition to carbonyls
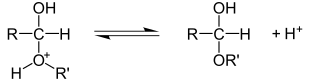
Addition reactions can also be carried out on carbonyl groups . In contrast to the addition to CC bonds, the CO bond is always polarized due to the higher electronegativity compared to carbon. Nucleophiles therefore always attack the electron-poor carbon atom, electrophiles consequently react with the oxygen atom. An example of an electrophilic addition to a carbonyl group is the formation of acetals from ketones or aldehydes . In the first step, the carbonyl oxygen is protonated by means of an acid. This creates a positive charge on the carbonyl carbon, which an alcohol attacks nucleophilically in the second step. The hemiacetal is released by splitting off the proton on the previous alcohol.
literature
- Peter Sykes: How do organic reactions work? 2nd edition, Wiley-VCH 2001, ISBN 3-527-30305-7
- Marye Anne Fox / James K. Whitesell: Organic Chemistry, Fundamentals, Mechanisms, Bioorganic Applications , Spectrum, Akad. Verl., 1995, ISBN 3-86025-249-6
Web links
- Electrophilic addition - explanations, examples, video
- Chempage: Electrophilic Addition - Good Explanation