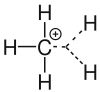
Carbocation
As carbocation in are organic chemistry hydrocarbon - molecules called that a positively charged carbon atom possess. With these molecules, a distinction is made between the classical carbocations with three substituents and the non-classical carbocations with five substituents. Carbocations with a three-coordinate carbon atom are called carbenium ions ( + CR 3 ; R stands for an organic radical ). The others are called carbonium ions . These are cations with a five-coordinate carbon atom (e.g. + CR 5 ). The term carbocation was introduced by George A. Olah , pioneering work on it comes from Hans Meerwein (from 1922).
Carbenium ions occur in organic chemistry as intermediates in so-called S N 1 reactions and in E 1 reactions. Reactions with the S N 1 mechanism belong to the nucleophilic substitutions , reactions with the E 1 mechanism belong to the class of the elimination reactions . Carbenium ions are sp 2 hybridized and thus trigonal planar.
The nucleophilic attack takes place on the empty p z orbital of the carbon. This is perpendicular (at right angles ) to the 3 hybrid orbitals , which means that a further substituent can attack from above or below. In general, S N 1 reactions therefore do not proceed stereoselectively . A carbenium ion is a trivalent carbon that has an electron sextet. Depending on the number of organic residues, a distinction is made between primary (+ CH 2 R), secondary (+ CHR 2 ) and tertiary carbenium ions (+ CR 3 ). The stability of the carbocations increases with the number of residues from primary to tertiary due to so-called I-effects and hyperconjugation .
Carbonium ions, on the other hand, only occur as a theoretical intermediate state in the S N 2 reactions. Here, the former nucleophile of the carbon atom is displaced by the new nucleophile attacking the back, creating this five-bonded carbonium ion as an intermediate state.
literature
- Armin de Meijere: News about carbocations. In: Chemistry in Our Time . 9th year 1975, No. 2, pp. 35-42, doi : 10.1002 / ciuz.19750090202
Individual evidence
- ↑ Entry on carbocation . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00817 Version: 2.1.5.