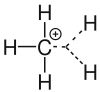
Carbenium ion
As carbenium ion in which is organic chemistry , a hydrocarbon - molecule referred to, the positively charged carbon atom has. Carbenium ion is a sub-term for carbocation . A carbenium ion is a three-bonded carbon that has an electron sextet and a free p orbital . A negatively charged carbon atom as part of an organic molecule is called a carbanion .
According to the number of organic residues, a distinction is made between primary ( + CH 2 R), secondary ( + CHR 2 ) and tertiary carbenium ions ( + CR 3 ). The stability of the carbenium ions increases in this order. If the radical R is a vinyl group or an aryl radical (e.g. phenyl radical ), the carbenium ion is mesomerically stabilized . The triphenylcarbenium ion is particularly stable. For the assessment of the relative stability of different carbenium ions, the mesomeric influences ( M-effect ) are more important than the inductive effects ( I-effects ) and hyperconjugation . If mesomerism stabilization is not possible, the stability of the carbenium ions increases with the number of radicals R from primary to tertiary due to the I effect.
Carbenium ions often occur as reactive intermediates in chemical reactions in organic chemistry, for example in the first-order nucleophilic substitution (S N 1 reaction). Carbenium ions can also compete with bromonium ions in bromine addition to the C = C double bond of alkenes :
They can be made with super acids such as antimony pentafluoride (SbF 5 ). When tert-butyl fluoride is reacted with antimony pentafluoride, heterolytic cleavage of the CF bond occurs with formation of the tert-butyl cation and the SbF 6 - anion . Both ions can form a stable salt that has a long shelf life at low temperatures. Tertiary alkylcarbenium ions can also be produced by splitting off a hydride ion from the corresponding hydrocarbon with the aid of a strong protic acid , such as a mixture of antimony pentafluoride and fluorosulfonic acid .
literature
- Armin de Meijere: News about Carbocations, Chemistry in Our Time , 9th year 1975, No. 2, pp 35-42, ISSN 0009-2851
Individual evidence
- ^ Vollhardt, C. et al: Organische Chemie, Wiley-VCH-Verlag, 4th edition, Weinheim 2005, page 284.
- ↑ Entry on carbenium ion . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00812 Version: 2.1.5.
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th edition, de Gruyter Verlag, Berlin, 2011, pp. 166–167, ISBN 978-3-11-024894-4 .