Carbonium ion
In organic chemistry, the carbonium ion is a special type of carbocation . The term was chosen in analogy to the ammonium ions . Carbonium ions are non-classical carbocations because they contain a five-bonded carbon atom. They are not to be confused with the carbenium ions , which have an electron sextet. In the literature, these terms have traditionally been used synonymously, but according to IUPAC, they must be delimited.
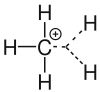
An example of carbonium ions would be CH 5 + , the methonium ion, which is formed, among other things, during the reaction of methane and fluorosulfonic acid or during chemical ionization in mass spectrometry. Since methane does not have a lone pair of electrons , the proton has to share an already bound electron pair with a carbon atom and a hydrogen atom. When formulating the valence bar formula for a protonated methane, the octet rule can only be preserved by forming a three-center bond .
In combination with strong super acids , 6-fold coordinated carbon in the form of CH 6 2+ is also possible. Since the reactivity of these protically activated compounds far exceeds the reactivity of the original electrophiles , these are referred to as superelectrophiles.
Individual evidence
- ↑ George A. Olah: Carbocations and Electrophilic Reactions . In: Angew. Chem. Vol. 85, No. 5 , January 1972, p. 183–225 , doi : 10.1002 / anie.19730850502 .
- ↑ Entry on carbonium ion . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00839 .
- ↑ George A. Olah: Superelectrophiles . In: Angew. Chem. Vol. 105, No. 6 , April 1992, pp. 805-827 , doi : 10.1002 / anie.19931050604 .