Inorganic chemistry
The Inorganic Chemistry (abbreviated AC ) or inorganic chemistry is the chemistry of all carbon-free connections and some exceptions (see Inorganics ). Organometallic compounds are a border area to organic chemistry . While organic chemistry only uses these as an aid or reagent, inorganic chemistry considers the coordination chemistry of metals.
Historically, inorganic chemistry dealt with substances that are not produced by organic life through life force . Since Friedrich Wöhler's urea synthesis in 1828 , in which the organic substance urea was produced from the inorganic compound ammonium cyanate , the boundaries between substances from inanimate (the "inorganic" substances) and living nature (the "organic" substances) have been blurring. Living things also produce a large number of inorganic substances, while almost all organic substances can now be produced in the laboratory. Nevertheless, the modern differentiation is still useful, since the reaction mechanisms and substance structures in inorganic and organic compounds differ widely.
History of inorganic chemistry
Many inorganic substances and some inorganic substance conversions were already known in ancient times. The metal recovery from ores ( gold , silver , copper , tin , lead , iron , mercury ), the pottery industry, the glass preparation (Egypt), the manufacture of porcelain (China), the mineral colors ( white lead , red lead , verdigris , cinnabar , Auripigment ), the Sulfur for smoking, lime (as mortar for residential buildings), salts such as table salt (for food preparation ), soda (for making glass and soaps ), saltpetre (as a remedy), alum (in tanneries).
In the alchemical age, in the 13th century, production methods for the extraction of sulfuric acid , dilute hydrochloric acid , nitric acid ( separating water to dissolve silver from gold-silver alloys) and aqua regia (hydrochloric and nitric acid to dissolve gold) were used by Arab alchemists (pseudo-donors Fonts). The manufacturing processes for acids were later significantly improved by Johann Rudolph Glauber around 1650, he also developed a process for the production of fuming hydrochloric acid.
In his main work The Skeptical Chemist, Robert Boyle described a move away from the Aristotelian theories of alchemy to experimental research and conclusions based on experiments. His thesis was significant that the chemical elements are composed of indivisible, identical, small atoms , chemical compounds of a multitude of small, different elements.

Georg Ernst Stahl and Johann Joachim Becher developed the phlogiston theory around 1700 . With this theory, which turned out to be incorrect 80 years later, combustion processes, oxidations and reductions as well as fermentation could be interpreted chemically. The cause of the misinterpretation of the phlogiston theory was a substance ( oxygen ) in the air that was still unknown at the time.
Joseph Priestley made studies with the air and realized that there is a substance in the air that promotes respiratory processes and promotes the oxidation of metals to metal oxides. By heating mercury (II) oxide , Priestley was able to extract the substance - which promotes breathing and combustion processes - in gaseous form and also determine the content of this substance in the air. It was only Antoine Laurent de Lavoisier who drew the conclusion from Priestley's findings that this newly discovered substance (oxygen) must be an element. Lavoisier's conclusions confirmed Boyle's theory of the elements and viewed the elements as a multitude of equal, indivisible atoms. A chemical compound contains several different elements. The metals gold, silver, copper, tin, lead, zinc and the non-metallic elements phosphorus , sulfur, carbon , oxygen and nitrogen were classified as pure elements . Lavoisier also recognized that the sum of the weights of the starting and end products remains the same for every substance conversion (law of conservation of mass ). The old, alchemical names of inorganic substances were changed by a rational name with the respective elementary building blocks of the mixture of substances. The oxidation theory according to Lavoisier represented a groundbreaking innovation in chemistry, the subsequent chemists now had to find the pure elements.
The discovery of electricity by Luigi Galvani and Alessandro Volta took place almost at the same time . Through this voltaic column , the water could be broken down into the elements oxygen and hydrogen gas and the composition of the water could be precisely determined by determining the volume and weight of the two gases.
Humphry Davy was able to separate sodium and potassium as new elements with the voltaic column . John Dalton put together a first - still very imprecise - atomic weight table for elements, Jöns Jacob Berzelius invented a method for determining the very exact atomic weights of metals and other elements and also developed the formula language with one or two Latin letters to identify the elements and related the relative atomic weights on oxygen as a reference point. Amedeo Avogadro put forward the hypothesis that the same number of particles of a gas must always be present in rooms of the same size and at the same temperature.
In the years that followed, the search for new chemical elements, the determination of their exact relative atomic weights and their characterization through reactions with other substances were among the most important tasks of inorganic chemists.
Joseph Louis Gay-Lussac developed titration and was able to determine the quantitative content of individual elements in an inorganic compound. Electrogravimetric separation was later used to determine the content of mineral samples. Robert Bunsen improved the method of generating electricity by developing a zinc-carbon battery . The new elements magnesium , chromium and strontium could be extracted in his laboratory . The spectral analysis developed by Bunsen led to the discovery of the elements cesium and rubidium , and later also helium by William Ramsay .
Lothar Meyer and Dimitri Mendelejew sorted the chemical elements according to their atomic weight and binding capacity in a periodic table . This made it easier to make predictions about the chemical behavior of elements and to look for elements that were still unknown in the system.
Svante Arrhenius , Jacobus Henricus van't Hoff and Wilhelm Ostwald recognized that the molecules of acids, bases and salts exist as ions in aqueous solutions . The discovery of the dissociation of salts and acids was the basis for important new findings (e.g. reaction mechanisms, kinetics) and measurement methods (e.g. pH measurement, conductometry) in chemistry.
Inorganic substances
The inorganic substances traditionally include the elements and all compounds that do not contain carbon .
There are also some exceptions to carbon compounds, which are built up exactly like typical inorganic substances or are historically assigned to inorganic substances. These include hydrogen-free chalcogenides of carbon ( carbon monoxide , carbon dioxide , carbon disulfide ), the carbonic acid and carbonates , the carbides and the ionic cyanides , cyanates and thiocyanates . The cyanide is considered as a limiting case and is treated in both the organic and inorganic chemistry. Although it would traditionally be counted as part of inorganic chemistry, it is regarded as the nitrile (organic group of substances) of formic acid.
Inorganic chemistry textbooks are organized according to the chemical elements of the periodic table . The textbooks deal with the occurrence and extraction of elements or element compounds from minerals, salts, aqueous solutions or gases. It also describes important conversions of these elements with other elements.
Metals
Of the hundred or so elements in the periodic table, 76% are metals . As early as 3000 to 2000 BC BC people extracted metals such as tin, copper, silver and iron from ores. Metals were obtained by heating mineral ores to a high temperature. With the exception of mercury, all metals are solid at room temperature and can be liquefied by heating. The good formability of liquefied metals for the production of everyday objects is still widely used today. Metal properties are the conductivity for heat and electricity .
Metals have been extracted using electricity ( electrolysis and electrolytic refining) since the 19th century . New metals - such as aluminum and the alkali and alkaline earth metals - were discovered. For many areas of application, light metals such as aluminum or titanium are required so that airplanes, automobiles, rail vehicles and machines do not consume excessive amounts of energy. Because of its high hardness and temperature resistance, iron is the most important metal in automobile construction. The surface of the iron tends to rust when exposed to moisture. In the early 1980s, many vehicles were still showing signs of rusting. Ten years later, many vehicles were already galvanized to protect against rust.
Metals are also used as batteries and accumulators to generate electricity. In inexpensive zinc-carbon batteries , the zinc is oxidized to the zinc salt while the electricity is being delivered. Other important batteries are nickel-metal hydride batteries (rechargeable!), Lithium batteries (very light battery) or the inexpensive lead-lead oxide accumulators (rechargeable!).
Alloys of metals can sometimes have better properties than the pure elements. The duralumin is a mixture of magnesium, copper and aluminum, it has a higher hardness than the pure aluminum. The Wood's metal is an alloy of bismuth, lead, cadmium and tin with a very low melting point, it is used for fusible forms. Other important metal alloys are bronze (made of copper and tin), brass (made of copper and zinc) and steel (iron alloys with various admixtures).
Some metals can combine with non-metals to form a crystal structure with new properties. The silicon combines with germanium , indium or arsenic . Such crystals are used as semiconductors (diodes and light emitting diodes) in electronics. Other metals - such as tantalum - are used as capacitors in electronics.
Metals or metal ions act as catalysts in reactions in the gas phase or in a liquid, for example iron in ammonia or aluminum ions in polyethylene synthesis.
Salts, minerals
The water is the main material of inorganic chemistry, it has covalent bonds between the polarized atoms and can accommodate many inorganic salts dissolve well. The temperature range between the freezing and boiling points of water enables life on our planet through the solubility of inorganic and organic substances in liquid water.
Inorganic salts differ in their solubility in water. Due to the different solubility in water, salts can be separated in many ways by filtration .
The mixture of two salts that are soluble in water - e.g. B. barium chloride and sodium sulfate - can lead to the formation of a poorly soluble salt (barium sulfate). If the solubility product of a salt is low, it precipitates.
Many metal cations combine with sulfide anions to form sulfides that are difficult to dissolve in a solution . With the right choice of acids and bases, certain groups of chemical elements can be enriched and determined as sulphide precipitates. In analytical chemistry, sulphide precipitation is an important separation process for metal cations.
Metal cations are also found in rocks and minerals. The metals in rocks are often in the form of silicates and these are not at all soluble in water. In addition to very strong acids, inorganic compounds use soda digestion to dissolve the constituents of the rocks.
Concrete : Silicates are very important, for example aluminum silicates, which are known as clay minerals . If you mix this clay with lime, you get cement. Mixing gravel and stone crushed stone with cement mortar creates concrete. Almost all residential buildings in Germany contain some concrete.
Porcelain : Another type of clay is kaolin . With quartz and feldspar, the clay is converted into porcelain by firing.
Glass : If quartz sand is heated at 1000 ° C by adding the salt soda, glass is produced.
Poorly soluble salts were and are also used as pigments for coloring paints.
Inorganic salts are very important as fertilizers . These salts are usually readily soluble in water. Sometimes too high a solubility is not always desirable in fertilization. Ammonium sulfate, potassium chloride and phosphate fertilizers (less water-soluble) increase the fertility of the soil considerably.
acids and bases
Very important inorganic acids are:
Important inorganic bases are:
The inorganic acids and bases are required for the extraction of inorganic salts and organic substances. In terms of quantity, sulfuric acid is the most important compound in the entire chemical industry. Inorganic acids break down metals into salts, and hydrogen is formed.
The knowledge about acids and bases has expanded considerably through the dissociation theory.
Gases

Many inorganic material conversions are associated with the development of gases. During the electrolysis of water, hydrogen and oxygen gas are evolved.
With chlor-alkali electrolysis , the gases hydrogen and chlorine are produced . These gases can react to form hydrogen chloride gas and hydrochloric acid is formed with water.
By burning sulfur in the air, the sulfur dioxide gas develops . A catalyst ( contact process with vanadium oxide) allows two sulfur dioxide molecules to react with another oxygen atom to form sulfur trioxide . The sulfur trioxide gas dissolves in water to form sulfuric acid.
Hydrogen sulfide can be produced from pyrite (FeS 2 ) and hydrochloric acid.
Carbon dioxide is produced by heating calcium carbonate when burning lime to make cement. When the cement hardens, it absorbs carbon dioxide from the air again.
From the nitrogen and hydrogen gas in the air, ammonia gas can be produced under high pressure and at 500 ° C using the Haber-Bosch process .
The ammonia can be combined with oxygen using the Ostwald process to form nitrogen monoxide, which reacts with additional oxygen to form nitrogen dioxide. The nitric acid then forms when dissolved in water.
Oxygen, nitrogen and argon can be extracted from the air using the Lind method . Processes for gas separation using very fine-pored membranes (gas membrane ) are also gaining increasing economic interest in separating individual gases .
Atmospheric chemistry is a very important field in the study of gases in the air .
Others
Inorganic cations can be present in different oxidation states as solid salts or in solution. As a result, they can also have different numbers of anions as counterions. In a solution, particles ( ligands ) with (e.g. chloride, thiocyanate) or without a negative charge (e.g. ammonia or water due to free electron pairs) can attach to cations and form colored complexes . Complexes are formed with more - for steric reasons often four to six - ligands on the cation than the oxidation number dictates. The ions of the transition metals ( titanium , vanadium , chromium , manganese , iron , cobalt , nickel , copper ), which have electrons on the d-shell, form multi-colored complexes with ligands. The copper (II) ion forms the blue colored copper tetramine complex with ammonia. In Berlin blue , iron (III) hexacyanoferrate, each iron ion is surrounded by six cyanide ions as ligands.
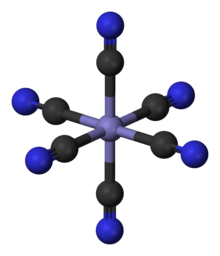
The ligand field theory describes the spatial coordination. With magnetochemistry and the color of the solution, inorganic chemists can make statements about the coordination of such complexes. In the permanganate anion, the manganese (VII) ion has four oxygen atoms as ligands. The well-colored complex of potassium permanganate is used for quantitative content determination in titrimetry.
Organic acids such as EDTA (quantitative determination of alkaline earth ions) or tartaric acid or citric acid (with copper (II) as Fehling's solution or Benedict's reagent for the determination of oxidizable sugars) and dioximes ( diacetyldioxime for nickel determination) are often suitable as coloring ligands (more precisely chelates ). for cations.
Inorganic reactions
A large number of reactions play a role in inorganic chemistry. The most important of these are the redox reactions and the acid-base reactions . These reactions are always equilibrium reactions, but the equilibrium in these reactions is often very much on one side and there is a high reaction enthalpy . As a result, many reactions in the inorganic are fast and achieve a high yield. In contrast, in organic chemistry, many reactions are slow equilibrium reactions that do not always achieve high yields.
In redox reactions, electrons are transferred from one reaction partner to the other. Typical redox reactions are reactions from elements to compounds. The best-known redox reactions are the oxyhydrogen reaction of hydrogen and oxygen to form water and corrosion , in which base metals (e.g. iron) react with oxygen to form oxides.
Acid-base reactions are reactions in which protons are transferred. The acid gives off a proton to the base (also: lye). Acid-base reactions usually form water and a salt (the best-known example is the reaction of hydrochloric acid with sodium hydroxide solution to form sodium chloride and water). Since these reactions take place very quickly and can be checked precisely with indicators, they play a major role in analytical chemistry .
In inorganic chemistry, the formation of insoluble salts or gaseous compounds is an important driving force for reactions, because reaction products leave the equilibrium and the reaction therefore goes completely in only one direction. When a barium chloride solution and plenty of sodium sulphate solution are poured together, barium sulphate that is very difficult to dissolve is precipitated in a precipitation reaction , so completely that after the barium sulphate has been filtered off, no more barium ions can be detected in the remaining sodium chloride solution :
An example of a directed equilibrium reaction due to escaping gases is the conversion of ammonium chloride with sodium hydroxide solution to volatile ammonia :
Such reactions also play an important role in analytical chemistry.
Various inorganic compounds can break down at higher temperatures by releasing gases. One example is lime burning , in which carbon dioxide escapes from calcium carbonate and calcium oxide remains.
Sub-areas of inorganic chemistry

- Chemistry of metals
- Chemistry of non-metals
- Complex chemistry , including bioinorganic chemistry
- Solid state chemistry
- Crystallography
- Structural chemistry
- Organometallic chemistry (stands between inorganic and organic chemistry)
- Colloid chemistry
- Atmospheric chemistry
- Mineral acids
Technical applications
Inorganic chemistry is the basis of various technical applications, for example
- Semiconductor chemistry
- mineralogy
- metallurgy
- Manufacture of cement , setting of mortar and concrete
- Manufacture of ceramics
Inorganic chemistry in school and university
school
In the field of inorganic chemistry, students learn the basics of atomic theory , the chemical behavior of elements , the oxidation numbers of the elements, the properties of inorganic salts (color reactions, solubilities), precipitation reactions, ion theory, acid-base reactions, and content determination by titration , Redox reactions, electrolysis and important technical processes.
Education
Independent scientific work in inorganic chemistry is taught during the course. The chemistry student gets to know the detection reactions for cations and anions from unknown samples. The practical course in inorganic-analytical chemistry is divided into the qualitative and quantitative analysis of inorganic substances. Furthermore, the preparative representation of inorganic substances is taught. The student learns to sharpen his powers of observation, to work carefully and to think methodically and combinatorially.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- DF Shriver, PW Atkins , CH Langford: Inorganische Chemie , 2nd edition. Wiley-VCH, Weinheim 1997, ISBN 978-3-527-29250-9 .
- J. Huheey, E. Keiter, R. Keiter: Inorganic chemistry - principles of structure and reactivity , 3rd edition. Walter de Gruyter, Berlin - New York 2003, ISBN 3-11-017903-2 .
- Lothar Kolditz (Hrsg.): Inorganikum - textbook and practical book of inorganic chemistry; with an introduction to physical chemistry. Deutscher Verlag der Wissenschaften, Berlin 1967, 12th edition 1989; Johann Ambrosius Barth Verlag, Leipzig-Berlin-Heidelberg, 13th edition 1993. Russian translation: Mir Verlag, Moskva 1984.
- Comments on Inorganic Chemistry
Exercise books
- Ehrhard Uhlemann, Gerhard Röbisch: Questions and tasks about chemistry . 3. Edition. VEB German publishing house of the sciences. Berlin 1988, ISBN 3-326-00275-0
See also
Web links
- Lecture Experimental Chemistry I, Inorganic Chemistry Video recordings of a lecture. From TIMMS, Tübingen Internet Multimedia Server of the University of Tübingen .