lead
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Lead, Pb, 82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 14 , 6 , p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Bluish white | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7439-92-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-100-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.273 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 18 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 207.2 (1) u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 180 (154) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 146 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 202 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 4 f 14 5 d 10 6 s 2 6 p 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.416 679 9 (6) eV ≈ 715.6 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 15th.032 499 (7) eV ≈ 1 450.42 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 31.9373 (6) eV ≈ 3 081.48 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 42.33256 (10) eV ≈ 4 084.47 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 68.8 (5) eV ≈ 6 640 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 11.342 g / cm³ (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −1.6 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 600.61 K (327.43 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 2017 K (1744 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 18.26 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 177 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 4.85 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 1260 m s −1 at 293.15 K. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 131 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 4.76 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 35 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson's number | 0.44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2 , 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −0.1251 V (Pb 2+ + 2 e - → Pb) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.33 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Authorization procedure under REACH | of particular concern : toxic for reproduction ( CMR ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lead is a chemical element with the element symbol Pb ( Latin plumbum ) and the atomic number 82. It is a poisonous heavy metal and is in the 4th main group or the 14th IUPAC group ( carbon group ) and 6th period of the periodic table . Lead is easy to deform and has a comparatively low melting point.
The isotopes 206 Pb, 207 Pb and 208 Pb are the heaviest stable atoms, so lead is the element with the highest mass and atomic number that is still stable. All lead isotopes have the magic proton number 82, which causes this stability. At 208 Pb there is even a so-called double magic nucleus because it also has the magic neutron number 126.
Since the lead isotopes -206, -207 and -208 are the end products of the three natural decay series of radioactive elements, a relatively large amount of lead was formed; it is therefore common in the earth's crust compared to other heavy elements ( mercury , gold, etc.).
history


The oldest find of metallic lead to date was made in Çatalhöyük , about 50 km southeast of Konya on the Anatolian Plateau. It consists of lead pearls together with copper pearls, which were dated to around 6500 BC.
In the early Bronze Age , lead was used alongside antimony and arsenic to produce bronzes from alloys with copper , until tin became widely accepted. The Babylonians were already familiar with lead vases. The Assyrians had to import lead ( abāru ), which is documented by Tiglath-pileser I., among other things, as a tribute from Melid . In ancient Greece , lead was mainly mined in the form of galena in order to extract silver. In the Roman Empire, however, the fabric was used for a variety of applications. Lead was of particular importance in architecture, for example, where blocks of stone were attached to one another with lead clips. An estimated seven tons of lead were used to build the Porta Nigra . Other important areas of application were the cladding of ship hulls to protect against pest infestation and the manufacture of inner-city water pipes. In addition, lead was used as a raw material for the production of vessels, as a material for writing boards or for the so-called tesserae , which were used, for example, as identification or authorization marks. Small pieces of lead, the so-called "sling lead", were used as slingshots in the Roman army . Due to the high demand, lead was also traded over long distances, which can be proven, among other things, by inscriptions on Roman lead ingots.
In ancient literature, it was felt, lead and tin are two manifestations of the same substance, so that one lead in Latin as plumbum nigrum (of niger , black ' ), tin as plumbum candidum (of candidus , white' hereinafter). It is therefore often unclear whether an ancient text by plumbum means lead or tin. Even the Roman author Vitruvius considered the use of lead for drinking water pipes to be harmful to health and recommended using clay pipes instead if possible. Nevertheless, drinking water pipes made of lead were in use until the 1970s, which is also expressed, for example, in the English word plumber , pipe laying . From today's point of view, the addition of lead as a sweetener to wine (so-called “lead sugar”, see lead (II) acetate ) was particularly worrying . The frequent use of lead in pipes and wine has also been discussed as a reason for the fall of the Roman Empire , but this is now rejected in research.
In Westphalia , the Romans gained lead until their retreat after the Varus Battle . The composition of the isotopes , which is typical for different sites, shows that the lead for the manufacture of Roman lead coffins found in the Rhineland comes from the northern Eifel . The Roman lead processing has led to a level of environmental pollution that can be traced to this day: ice cores from Greenland show between the 5th century BC. BC and the 3rd century AD a measurable increase in the lead content in the atmosphere.
Lead (from Middle High German blī ) also had an important meaning later . It was used, for example, for framing lead glass windows , for example in churches or for covering lead roofs . Lead became particularly important after the invention of firearms for the military as a material for projectiles for handguns. Since soldiers made their own projectiles, it was not uncommon for them to steal whatever lead they could find to make projectiles from it.
Lead also played an important role in alchemy . Due to its similarity to gold (similarly soft and heavy), lead was considered a good starting material for gold synthesis (synthesis as a color change from gray to yellow). The alchemical symbol for lead is a stylized sickle (♄), as it has been assigned to the god and planet Saturn as a planetary metal since ancient times .
With the beginning of the industrial revolution , lead was then required in large quantities for the chemical industry , for example for the production of sulfuric acid in the lead chamber process or the lining of plants for the production of explosives . It was the most important non-ferrous metal at the time .
In an attempt to determine the age of the earth by measuring the ratio of lead to uranium in rock samples, the American geochemist Clair Cameron Patterson found around 1950 that the rock samples were all contaminated with large amounts of lead from the atmosphere. He was able to identify the source of the tetraethyl lead used as an anti-knock agent in fuels . Before 1923, Patterson found that the atmosphere contained almost no lead at all. Based on this knowledge, he spent his life fighting to reduce the release of lead into the environment. His efforts eventually led to the 1970 US Clean Air Act with stricter emissions regulations. In 1986 the sale of leaded petrol was banned in the United States, gradually in Germany by the lead gas law from 1988, and completely banned in the EU from 2001. As a result, the level of lead in Americans' blood dropped 80 percent almost immediately. However, since lead remains practically forever in the environment, every person today has about 600 times more of the metal in their blood than before 1923. Around the year 2000, around 100,000 tons were still legally released into the atmosphere each year. The main polluters are mining, metal industry and manufacturing.
In 2009, the amount of lead extracted from non-ferrous metals was fourth after aluminum , copper and zinc . It is mainly used for car batteries ( lead accumulators ) (60% of total production).
In general, attempts are being made to reduce the exposure of people and the environment to lead and thus lead poisoning . In addition to the ban on leaded petrol, the use of lead in electrical and electronic equipment was restricted in 2002 by the RoHS guidelines . In 1989, paints and coatings containing lead were banned completely, and the use of ammunition containing lead was partially banned in some federal states from 2005. Lead was banned as a material for water pipes as early as 1973, but there is still no provision for the removal of lead pipes from existing properties, which is why the German Federal Council called for a ban on lead-containing drinking water pipes in 2017. Since March 1, 2018, the use (storage, mixing, use for production, etc.) and placing on the market of lead - solid (e.g. as bars or pellets) or as powder - has been similar to that of many lead compounds in the European Union With a few exceptions, it is regularly prohibited if it is intended for sale to the general public and the lead concentration therein is 0.3% or more; In addition, the supplier must ensure that this is marked as "only for commercial users" before it is placed on the market.
Occurrence
Lead occurs in the earth's crust with a content of around 0.0018% and occurs rarely in solid form , i.e. in elemental form. Nevertheless, around 200 sites for solid lead are known worldwide (as of 2017), including in Argentina , Ethiopia , Australia , Belgium , Brazil , People's Republic of China , Germany , Finland , France , Georgia , Greece , Greenland , Italy , Canada , Kazakhstan , Kyrgyzstan , Mexico , Mongolia , Namibia , Norway , Austria , Poland , Russia , Sweden , Slovenia , the Czech Republic , Ukraine , the US Virgin Islands , the United Kingdom and the United States of America (USA).
Lead could also be found in rock samples from the mid-Atlantic ridge , more precisely on the northeastern edge of the “Markov Depth” within the “Sierra Leone Fracture Zone” (Sierra Leone Threshold), as well as outside the earth on the moon in Mare Fecunditatis .
At each location, the isotope composition differs slightly from the mean values given above, so that a precise analysis of the isotope composition can determine the location and, in the case of archaeological finds, draw conclusions about old trade routes. In addition, depending on where it is found, lead can also contain various foreign admixtures such as silver, copper, zinc, iron, tin and / or antimony.
In lead ores, lead is mostly present as galena (lead sulfide PbS, lead luster ). This mineral is also the most important commercial source for the extraction of new lead. Other lead minerals are cerussite (lead (II) carbonate, PbCO 3 , also white lead ore ), crocoite (lead (II) chromate, PbCrO 4 , also red lead ore ) and anglesite (lead (II) sulfate, PbSO 4 , also lead nitride ) . The lead minerals with the highest lead concentration in the compound are lithargite and massicotite (up to 92.8%) and minium (up to 90.67%). A total of 514 lead minerals are known to date (as of 2017).
The economically minable stocks are estimated at 67 million tons worldwide (status 2004). The largest deposits are found in the People's Republic of China , the USA , Australia , Russia and Canada . In Europe, Sweden and Poland are the countries with the largest deposits.
In Germany, too, in the northern Eifel (Rescheid / Gruben Wohlfahrt and Schwalenbach; Mechernich / Grube Günnersdorf and also open-cast mining / Virginia; Bleialf ), in the Black Forest, in the Harz (Goslar / Rammelsberg ), in Saxony (Freiberg / Muldenhütten ), at the lower Lahn ( Bad Ems , Holzappel ), as well as in Westphalia ( Ramsbeck / Sauerland) in the past lead ore mined, smelted and refined.
The most important source of lead today is the recycling of old lead products. Therefore, there are only two primary smelters left in Germany that produce lead from ore, the Binsfeldhammer lead smelter in Stolberg (Rhld.) And Metaleurop in Nordenham near Bremerhaven . All other smelters produce so-called secondary lead by processing old lead (especially from used car batteries ).
Lead as a mineral
Natural occurrences of lead in its elementary form were known even before the International Mineralogical Association (IMA) was founded. The manganese-rich iron ore deposit Långban in Sweden is given as a presumable type locality , where massive masses of up to 50 kg or 60 kg are said to have been found. Lead is therefore recognized as a so-called grandfathered mineral as an independent type of mineral.
According to the systematics of minerals according to Strunz (9th edition) , lead is listed under the system number 1.AA.05 (elements - metals and intermetallic compounds - copper cupalite family - copper group) or in the outdated 8th edition under I / A.03 ( tin-lead group ) classified. The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. 01/01/01/04 ( gold group ).
In nature, solid lead occurs mostly in the form of centimeter-sized sheets and plates as well as granular, dendritic, hair-shaped or wire-shaped aggregates . Very rarely, there are also octahedral , diced and dodecahedral lead crystals , which are usually tiny, but occasionally a size between 4 cm and 6 cm reach.
States with the greatest funding
| rank | country | Delivery rates (in 1000 t ) |
rank | country | Delivery rates (in 1000 t) |
|---|---|---|---|---|---|
| 1 |
|
950 | 11 |
|
33.9 |
| 2 |
|
642 | 12 |
|
33 |
| 3 |
|
445 | 13 |
|
31.3 |
| 4th |
|
306.2 | 14th |
|
24 |
| 5 |
|
118.5 | 15th |
|
22nd |
| 6th |
|
76.7 | 16 |
|
20th |
| 7th |
|
65.9 | 17th |
|
19th |
| 8th |
|
39.8 | 18th |
|
18.7 |
| 9 |
|
38 | 19th |
|
15th |
| 10 |
|
37.5 | 20th |
|
14.7 |
The world's most important producers of lead ore in 2004 were the People's Republic of China (950,000 tons), Australia (642,000 tons) and the USA (445,000 tons), which together accounted for around two thirds of the 3.1 million tons mined worldwide. In Europe, Ireland, Sweden and Poland are the largest lead producers.
The most important producers of refined lead ( metallurgical soft lead with 99.9% purity) are the People's Republic of China (1.8 million tons), the USA (1.2 million tons) and Germany (403,000 tons), which together make up about half of the 6.7 million tons produced worldwide. Other major producers of refined lead in Europe are Great Britain, Italy, France and Spain.
The global consumption and production of lead rose from around 7 million tons to around 11 million tons in the years 2013 to 2016.
Extraction and presentation
By far the most important lead mineral is galena . This often occurs in association with the sulphides of other metals (copper, bismuth, zinc, arsenic, antimony, etc.), which naturally contain up to 5% of the raw lead as an impurity.
The ore, which has been processed to a mineral content of up to 60% through crushing, classification and flotation , is converted into metallic lead in three different industrial processes. The processes of roasting reduction and the roasting reaction are increasingly taking a back seat and are being replaced by direct melting processes, which on the one hand can be made more economical and on the other hand are more environmentally friendly.
Roast reduction work
This process takes place in two stages, roasting and reduction . When roasting, the finely ground lead sulphide is placed on a traveling grate and hot air at 1000 ° C is forced through it. It reacts with the oxygen in the air in an exothermic reaction to form lead (II) oxide (PbO) and sulfur dioxide . This is expelled via the roasting gases and can be used for sulfuric acid production. The lead oxide is liquid under these conditions and flows downwards. There it can be sintered .
- (Roasting work)
The lead oxide is then reduced to metallic lead with the aid of coke . This is done in a shaft furnace, similar to that used in the blast furnace process. Slag-forming additives such as lime are added.
- (Reduction work)
Roast reaction work
This process is mainly used for lead ores that are highly enriched with PbS and enables lead generation in one step. The sulfidic ore is only roasted incompletely. The lead sulfide / lead oxide mixture is then heated further in the absence of air. The lead oxide reacts with the remaining PbS to form lead and sulfur dioxide without adding any other reducing agent:
- (Roasting work),
- (Reaction work).
Direct melting process
Modern lead manufacturing processes are based on direct smelting processes that have been optimized for environmental compatibility and economic efficiency (e.g. the QSL process ). The continuous process management with restriction to one reaction chamber, which is the only emitter for pollutants, is advantageous - in comparison, the classic production processes have sintering as an additional emitting step. Roasting and reduction take place in parallel in one reactor. The lead sulfide is not completely roasted, similar to the roasting reaction process. Part of the lead is thus produced by the reaction of the lead sulfide with lead oxide. Since the reactor is slightly inclined, lead and lead oxide-containing slag drain off. This passes through the reduction zone, into which coal dust is blown and the lead oxide is reduced to lead. When roasting, pure oxygen is used instead of air . This significantly reduces the volume of exhaust gases, which on the other hand have a higher concentration of sulfur dioxide compared to conventional processes. Their use for the production of sulfuric acid is therefore simpler and more economical.
Refining
The resulting lead contains 2–5% other metals, including copper , silver , gold , tin , antimony , arsenic and bismuth in varying proportions. The purification and marketing of some of these by-products, especially the silver contained in up to 1% of the lead, contributes significantly to the profitability of lead extraction.
The pyrometallic refining of lead is a multi-stage process. By melting in the presence of sodium nitrate / sodium carbonate or air, antimony, tin and arsenic are oxidized and can be removed from the surface of the molten metal as lead antimonates, stannates and arsenates (“antimony smear”). Copper as well as possibly contained zinc, nickel and cobalt are removed from the raw metal by segregating the lead. The sulfur content also drops considerably. According to the Parkes process, silver is deposited from the lead (“Parkesization”) by adding zinc and the segregation of the Zn-Ag mixed crystals that are formed, while the importance of the older Pattinson process has declined significantly ( see also production of silver, look silver ). According to the Kroll-Betterton process, bismuth can be stripped from the surface of the molten lead as a bismuth foam by alloying with calcium and magnesium .
Further purification can be done by electrolytic refining, but this process is more costly due to the high energy requirements. Lead is a non-noble element, which has a more negative standard potential than hydrogen in the electrochemical series . However, this has a high overvoltage at lead electrodes , so that an electrolytic deposition of metallic lead from aqueous solutions is possible, see electrolytic lead refining .
Refined lead comes on the market as soft lead or standardized smelting lead with 99.9 to 99.97% purity (e.g. Eschweiler Raffiné ) or as fine lead with 99.985 to 99.99% lead (DIN 1719, outdated). Depending on the intended use, designations such as cable lead are also common for the alloy with approx. 0.04% copper. Current standards such as DIN EN 12659 no longer recognize these terms, which are still in use.
properties
Physical Properties
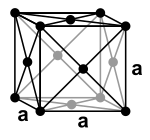
Lead is a base metal with a standard electrode potential of around −0.13 V. However, it is more noble than many other common metals such as iron, zinc or aluminum. It is a diamagnetic heavy metal with a density of 11.3 g / cm³, which crystallizes in a face-centered cubic manner and thus has a cubic closest packing of spheres with the space group Fm 3 m (space group no. 225) . The lattice parameter for pure lead is 0.4950 nm (corresponds to 4.95 Å ) with 4 formula units per unit cell .
This is the basis of the pronounced ductility of the metal and the low Mohs hardness of 1.5. It can therefore easily be rolled into sheet metal or shaped into wires, which are, however, only slightly resistant due to their low hardness. A diamond-like modification, as is known from the lighter homologues of group 14, does not occur with lead. This is due to the relativistic instability of the Pb-Pb bond and the low tendency to appear tetravalent.
Fresh lead samples are gray-white to metallic-white in color and show a typical metallic sheen , which, however, decreases very quickly due to superficial oxidation. The color changes into dark gray and becomes matt. The soft metal leaves a (lead) gray line on paper . For this reason people used to write and paint with lead. The name “ pencil ” has been used to this day, although graphite has been used for it for a long time .
The melting point of lead is 327 ° C, its boiling point is 1740–1751 ° C (values vary in specialist literature: 1740 ° C, 1746 ° C, 1751 ° C). As a typical metal, lead conducts both heat and electricity , but this is significantly worse than other metals (cf. electrical conductivity of lead: 4.8 · 10 6 S / m, silver: 62 · 10 6 S / m). Below 7.196 K, lead shows no electrical resistance , it becomes a type I superconductor . The speed of sound in lead is around 1200 m / s, in the literature the values vary somewhat, probably due to differences in purity or processing.
Chemical properties
In the air, lead is passivated by the formation of a layer of lead oxide and thus protected from further oxidation . Fresh cuts therefore initially have a metallic sheen, but quickly tarnish to form a matt surface. In a finely divided state, lead is highly flammable ( pyrophoric lead ).
Due to its passivation, lead is also insoluble in various acids. Lead is resistant to sulfuric acid , hydrofluoric acid and hydrochloric acid , since insoluble lead salts are formed with the anions of the respective acid. This is why lead has a certain importance in chemical apparatus engineering for special applications.
Lead, on the other hand, is soluble in nitric acid ( lead (II) nitrate is water-soluble), hot, concentrated sulfuric acid (formation of the soluble Pb (HSO 4 ) 2 complex), acetic acid (only with air access) and hot alkalis .
Metallic lead is stable in water that does not contain oxygen. In the presence of oxygen, however, it slowly dissolves, so that lead drinking water pipes can pose a health hazard. If, on the other hand, the water contains many hydrogen carbonate and sulphate ions , which is usually associated with high water hardness , a layer of basic lead carbonate and lead sulphate will form after a while . This protects the water from the lead, but even then some lead from the pipes will migrate into the water.
Isotopes
Naturally occurring lead consists on an average of about 52.4% of the isotope 208 Pb, about 22.1% of 207 Pb, about 24.1% of 206 Pb and about 1.4% of 204 Pb. The composition is slightly different depending on the deposit, so that the origin of the lead can be determined by analyzing the isotopic composition. This is important for historical finds made of lead and findings from earlier trade relations.
The first three isotopes mentioned are stable. At 204 Pb is a primordial radionuclide . It decays into 200 Hg with the emission of alpha radiation with a half-life of 1.4 · 10 17 years (140 quadrillion years) . 208 Pb has a doubly magical core ; it is the heaviest stable nuclide. (The more serious, long for stable held 209 Bi is unstable, according to recent measurements, and decays with a half-life of (1.9 ± 0.2) × 10 19 years (19 quintillion years) with emission of alpha particles in 205 Tl being. Very slow decay is due to the fact that with Z = 83 it has only one proton more than the magic proton number of 82 and the magic neutron number 126, i.e. it is very similar in structure to the doubly magic lead core with 208 nucleons).
The stable isotopes of naturally occurring lead are the end products of the uranium and thorium decay series: 206 Pb is the end nuclide of the uranium-radium series starting at 238 U , 207 Pb is the end of the uranium-actinium series starting at 235 U and 208 Pb the end of the thorium series beginning at 244 Pu and 232 Th, respectively . This series of decays has the effect that the ratio of the lead isotopes in a sample is not constant over time if a material exchange with the environment is excluded. This can be used to determine the age using the uranium-lead or thorium-lead method, which, due to the long half-lives of the uranium and thorium isotopes, in contrast to the radiocarbon method, is particularly suitable for dating samples that are millions of years old. In addition, the effect leads to differentiated isotope signatures in the lead from different deposits, which can be used for proof of origin.
There are also 33 unstable isotopes and 13 unstable isomers from 178 Pb to 215 Pb, which were either produced artificially or occur in the decay series of uranium or thorium , such as 210 Pb in the uranium-radium series. The longest-lived isotope among them is 205 Pb with a half-life of 153 million years.
use
The largest consumers of lead are the USA, Japan, Germany and the People's Republic of China. Consumption is heavily dependent on the economy in the automotive industry, in whose accumulators around 60% of the world's lead is used. Another 20% is processed in the chemical industry.
Radiation shielding

Because of its high atomic mass , lead in sufficiently thick layers or blocks is suitable for shielding against gamma and X-rays ; it absorbs X-rays and gamma rays very effectively. Lead is cheaper and easier to process for this purpose, for example as soft sheet metal, than even “atom-heavier”, denser metals. That is why it is generally used for shielding in radiation protection (e.g. nuclear medicine , radiology , radiation therapy ) . One example is the lead apron that doctors and patients wear when taking x-rays. Lead glass is also used for radiation protection.
In the hospital sector, the technical specification for structural facilities with a shielding function such as walls, doors, windows, is the lead thickness equivalent and is often written in order to be able to calculate the effectiveness of radiation protection and radiation exposure.
Lead is therefore z. B. also used for anti-scatter grids .
The shielding of gamma spectrometers for precision dosimetry is a special application . Lead with the lowest possible radioactivity is required for this. The natural content of radioactive 210 Pb has a disruptive effect. It turns out to be lower, the longer in the past since the time of smelting, because during smelting the mother nuclides from the uranium-radium series (companions in the ore) are separated from the lead. The 210 Pb therefore decays from the time of smelting on with its half-life of 22.3 years without any new replicas. This is why historical lead objects such as trim weights from sunken ships or historical cannon balls for the extraction of low-radiation lead for the production of such shields are in great demand. There are also other research institutions that need this old lead for similar reasons.
metal
Lead is mainly used as a metal or alloy . In contrast to earlier times, when lead was one of the most important and most widely used metals, attempts are now being made to replace lead with other, non-toxic elements or alloys. Because of its important properties, especially its corrosion resistance and high density as well as its ease of manufacture and processing, it is still of great importance in industry. Elements with a similar or even higher density, for example, are either more problematic ( mercury , uranium ) or very rare and expensive ( tungsten , gold , platinum ).
Electrical engineering
Most of the lead is now used for chemical energy storage in the form of lead-acid batteries (e.g. for cars). A car battery contains a lead and a lead (IV) oxide electrode and dilute sulfuric acid (37%) as an electrolyte. Insoluble lead (II) sulfate is formed in the sulfuric acid from the Pb 2+ ions produced in the electrochemical reaction . Recharging is possible through the reverse reaction of lead (II) sulphate to lead and lead (IV) oxide. One advantage of the lead accumulator is the high nominal voltage of a battery cell of 2.06 volts .
mechanical engineering
Since lead has a high density, it is used as a weight. Colloquially there is therefore the term "lead heavy" for very heavy things. Lead weights were used, among other things, as counterweights for balancing car wheels. However, this has been prohibited for new cars since July 1, 2003 and for all cars (up to 3.5 t) since July 1, 2005; the lead weights have been replaced by zinc or copper weights. Other applications that make use of the high density are: Lead chains for tightening curtains and diving weights to compensate for the buoyancy of the diver and equipment while diving. In addition, lead is used as a vibration damper in vibration-sensitive (car) parts, to stabilize ships and for special applications in noise protection.
Apparatus construction
Lead is chemically very resistant due to passivation and can withstand a. Sulfuric acid and bromine . It is therefore used as corrosion protection in apparatus and container construction. A previously important application was the lead chamber process for the production of sulfuric acid, since at that time lead was the only known metal that withstood sulfuric acid vapors. Earlier plants and rooms for the production of nitroglycerin were also lined with lead on the floor and wall. Lead has also been widely used to sheath cables to protect them from environmental influences, such as telephone cables. Today lead is mostly used in plastics , e.g. B. PVC, has been replaced, but is still used today in cables in refineries because it is also insensitive to hydrocarbons .
Construction

Because lead is easy to work and cast, lead has been widely used for metallic objects in the past. The most important lead products included a. Tube. Due to the toxicity of the chemical compounds that may arise from the lead ( lead poisoning ), lead pipes have not been used since the 1970s. Despite a carbonate layer formed in the pipes, the lead continues to dissolve in the drinking water . Experience has shown that the limit value of the applicable drinking water ordinance is no longer complied with after a few meters .
Lead was also used in building construction to connect stones with cast metal clips or metal dowels, for example to attach hinges to a stone door frame or an iron railing on a stone staircase. This lead technique is still widely used in restoration. At the top of the tower in St. Stephen's Cathedral in Vienna or the bridge in Mostar . Also for window frames, e.g. B. on medieval church windows, lead rods were often used. Lead (rolled lead) is also used as roofing (e.g. the main domes of Hagia Sophia ) or for roof closures (e.g. in the famous " lead chambers ", the former prison of Venice and in Cologne Cathedral) and to surround roof openings . In the past, lead was also mixed in with paints and anti-corrosive paints, especially in paints for metal surfaces. Even today, lead is a building pollutant that has to be taken into account in existing buildings , as it can still be found in many older building and plant parts.
Pneumatic controls
From the late 19th century onwards, a special area of application for lead pipes was pneumatic controls for organs ( pneumatic action ), pneumatic art play pianos and, as a special and very successful application, the control of the link trainer , the first widely used flight simulator. The advantages of lead pipes (cheap, stable, flexible, small space requirement for the necessary extensive pipe bundles, solderable, mechanically easy to process, durable) were decisive.
Military technology
The military was and is an important customer for lead metal. Lead is used as a raw material for projectiles, both for slingshots and for firearms. Chopped lead was shot in so-called grapefruit . The reason for the use of lead was and is, on the one hand, its high density and thus high penetration power and, on the other hand, its easy production by casting. Nowadays the lead is usually enclosed in a jacket (hence “ jacket bullet ”) made of a copper alloy ( tombak ). The main advantages are the higher bullet speed that can be achieved, at which an uncovered lead bullet can no longer be used due to its softness, and the prevention of lead deposits inside the barrel of a firearm. However, lead-free ammunition is also available.
Body repairs
Before the advent of modern 2-component fillers , lead or lead-tin alloys were used to fill damaged and repaired areas on vehicle bodies due to their low melting point. For this purpose, the material was soldered onto the damaged area with a soldering torch and flux . The place was then sanded down as with a spatula. This has the advantage that, in contrast to filler, the lead forms a firm bond with the sheet metal and, in the event of temperature fluctuations, also takes part in its linear expansion. Since the vapors and dust produced are poisonous, this process is rarely used today, except for the restoration of historic vehicles.
regional customs
An old oracle custom that the Romans already used is lead pouring , in which liquid lead (nowadays also in alloy with tin) is made to solidify in cold water. With the help of the randomly arising forms, prophecies about the future are to be made. Today the custom is still practiced at New Year's to get a (not necessarily taken seriously) outlook on the coming year.
water sports
When diving , lead weights are used for taring ; the high density excess (a good 10 g / cm³) compared to water provides compact downforce so that a diver can float even in shallow water. The comparatively low price is also beneficial for the use of lead as weight: based on the world market prices for metals from July 2013, lead has an excellent price-weight ratio. They are used in the form of plates on the soles of an armored diving suit, as rounded blocks threaded onto a wide hip belt or - modern - as shotgun pellets in nets in the pockets of a buoyancy compensator. Opening the belt buckle or the pockets (below) allows the ballast to be thrown off quickly if necessary.
The keel ballast of sailing yachts is preferably made of lead. Scrap iron is cheaper, but also less dense, which is not ideal with today's slim keels. In addition to the density, another advantage is that lead does not rust and therefore does not degenerate even if the keel cladding is damaged.
Alloy component
Lead is also used in some important alloys . By adding other metals to the alloy , the hardness , melting point or corrosion resistance of the material change depending on the metal . The most important lead alloy is hard lead , a lead-antimony alloy that is considerably harder and therefore more mechanically resilient than pure lead. Traces of some other elements (copper, arsenic, tin) are mostly contained in hard lead and also have a decisive influence on hardness and strength. Hard lead is used, for example, in apparatus engineering, where stability is important in addition to chemical resistance.
Another lead alloy is letter metal , a lead alloy with 60–90% lead, which also contains antimony and tin. It is used for letters in traditional letterpress printing , but today it no longer plays a role in the mass production of printed matter, but rather for bibliophile editions. In addition, lead is used in bearings as a so-called bearing metal .
Lead plays a role as an alloy component in soft solder , which is used, among other things, in electrical engineering . In addition to lead, tin is the most important component in soft solders. The use of lead in solders in 1998 was around 20,000 tons worldwide. The EU directive 2002/95 / EG RoHS has largely banned lead from soldering technology since July 2006 . However, there are a number of exceptions for special applications.
Lead is a common minor component in brass . There a lead content (up to 3%) helps to improve machinability. Also in other alloys, such as B. gunmetal , lead may be included as a minor component. It is therefore advisable not to drink the first water that comes out of the brass fittings after standing for a long time, because some lead has been dissolved out.
lead-free
Products and applications containing lead are either completely replaced (such as tetraethyl lead in gasoline ) or the lead content is limited to a level corresponding to the technical contamination by means of limit values (e.g. tin and solder ). These products are often called "lead-free". There are limit values a. in the legislation on the so-called RoHS (Directive 2011/65 / EU), which provides 1000 ppm (0.1%). The limit value for packaging is stricter with 100 ppm (Directive 94/62 / EC).
The political will to replace lead also applies where the use would be technically or economically interesting due to the properties, the health risk would be low and recycling would be possible with reasonable effort (e.g. lead as roofing).
Lead glass
Because of the shielding effect of the lead consists of the cone of cathode ray tubes (i. E. The "rear" part of the tube) for television , computer screens , etc. of lead glass . The lead absorbs the soft X- rays that inevitably arise in cathode ray tubes . For this purpose, lead cannot yet be safely replaced, so the RoHS directive is not applied here. Because of this shielding effect, glass with a very high lead content is also used in radiology and radiation protection (for example in window panes). Furthermore, because of its high refractive index, lead glass is used as a so-called lead crystal for high-quality glassware .
toxicity
Elemental lead can mainly be absorbed through the lungs in the form of dust. In contrast, lead is hardly absorbed through the skin. Therefore, elemental lead in compact form is not toxic to humans. Metallic lead forms a dense, poorly water-soluble protective layer of lead carbonate in the air. Dissolved lead compounds and lead dust that can get into the body through ingestion or inhalation are toxic. Organic lead compounds are particularly toxic, e.g. B. Tetraethyl lead , which are highly lipophilic and are quickly absorbed through the skin.
Inhalable fractions of lead and inorganic lead compounds have been classified as carcinogenic by the MAK Commission of the German Research Foundation since 2006 :
- Lead arsenate and lead chromate in Category 1 (“Substances which cause cancer in humans and which can be assumed to contribute to the risk of cancer. Epidemiological studies provide sufficient evidence for a connection between exposure in humans and the occurrence of cancer. "),
- Lead and other inorganic lead compounds except lead arsenate and lead chromate in category 2 ("Substances that are to be regarded as carcinogenic for humans because sufficient results from long-term animal experiments or indications from animal experiments and epidemiological studies can be assumed to contribute to the Risk cancer. ").
Lead accumulates in the body even with the ingestion of the smallest amounts, which are constantly ingested over a long period of time, as it is e.g. B. stored in bones and is only very slowly excreted. Lead can cause chronic poisoning, which manifests itself in headaches, tiredness, emaciation and defects in blood formation, the nervous system and muscles. Lead poisoning is particularly dangerous for children and pregnant women. It can also cause fruit damage and the inability to conceive. In extreme cases, lead poisoning can lead to death. The toxicity of lead is based, among other things, on a disturbance in hemoglobin synthesis . It inhibits several enzymes and thereby hinders the incorporation of iron into the hemoglobin molecule. This disrupts the oxygen supply to the body cells.
Lead glass and lead glaze are not suitable for eating and drinking vessels because acetic acid can dissolve lead as water-soluble lead acetate from the silicate compound. When car engines ran on petrol with tetraethyl lead, the vegetation near roads and in cities was contaminated with lead, as oxide dust. Rough and recessed surfaces, such as the indentation around the stem of an apple, are traps for dust.
Lead pollution of the environment
air
The lead pollution of the air is mainly caused by lead-containing dust : the main sources are the lead-producing industry, the combustion of coal and, until a few years ago, mainly car traffic due to the combustion of leaded fuels in car engines - halogenated hydrocarbons added to the gasoline resulted from this the added tetraethyl lead, as well as smaller amounts of lead (II) chloride and lead (II) bromide , especially lead and lead (II) oxide . As a result of the ban on leaded fuels, the corresponding air pollution has decreased significantly in recent years.
Lead exposure from lead dust is currently highest when working in lead-producing and processing companies. Old well in cleaning and removing red lead -Anstriche by sandblasting creates lead dust. The lead oxide dusts produced during lead refining and the burning of coal could be reduced by using suitable filters. Another source, which in terms of quantity is hardly significant, is the incineration of household waste in waste incineration plants .
Sports and other shooters are exposed to considerable loads from (heavy) metals contained in the muzzle or ignition light , including lead in addition to antimony , copper and mercury ; Precautions can be taken by operating appropriate extraction systems on shooting ranges and by using lead-free ammunition .
ground
Floors can also be contaminated with lead. The mean lead content of the continental crust is 15 mg / kg. Soils naturally contain between 2 and 60 mg / kg lead; if they are formed from rocks containing lead ore, the content can be significantly higher. Most of the lead contamination of soils is anthropogenic , the sources for this are diverse. The majority of the entry occurs via lead dust from the air, which gets into the soil with the rain or through dry deposition. For Germany and the year 2000, the atmospheric input into the soil was estimated at 571 t lead / year. Another source is loaded fertilizer , both mineral fertilizer (136 t Pb / a), in particular nitrate , as well as manure (182 Pb t / a). Sewage sludge (90 t Pb / a) and compost (77 t Pb / a) also contribute to the lead pollution of the soil. A significant entry also occurs from lead shot ammunition. In the case of contaminated sites, such as B. At former locations of lead-producing industrial plants or in the vicinity of old lead-sheathed cables, the floor can also have a high level of lead pollution. A particularly large lead contamination has, for example, led to high levels of pollution in children in the town of Santo Amaro da Purificação ( Brazil ).
water
The lead pollution of waters results mainly from the washout of lead from polluted soils. Small amounts that the rain loosens from lead materials such as roofing panels also contribute to this. The direct pollution of waters by the lead industry and lead mining plays (at least in Germany) almost no role anymore due to the construction of sewage treatment plants . The annual discharge of lead into water has decreased in Germany from around 900 t in 1985 to around 300 t in 2000.
In Germany, the limit value in drinking water has been 10 µg / l since December 1, 2013 (previously 25 µg / l); The measurement is based on a sample representative of the average weekly water intake by consumers (see Drinking Water Ordinance ).
food
Lead contamination of the air, soil and water causes the metal to enter the human food chain via fungi, plants and animals. Various mushrooms can contain particularly high levels of lead. Lead is deposited on the leaves of plants as dust, which was characteristic of the area around roads with a lot of motor vehicle traffic when gasoline was still leaded. This dust can be removed by careful washing. Additional sources can be lead ammunition from hunted animals. Lead can also migrate into food from lead-containing glazes on ceramic vessels. In the vast majority of cases, lead and cadmium cannot be detected in fresh fruit and vegetables, or only in very small traces.
Water pipes made of lead pipes can pollute drinking water. They have not been installed in Germany until the 1970s. Lead pipes are still to be found, especially in old buildings in some regions of northern and eastern Germany. In over 5% of the samples of the water from these buildings, the lead values in the tap water were above the current legal limit, according to Stiftung Warentest . The same applies to Austria and concerns house supply lines of the water supplier and lines in the house, which are the responsibility of the house owner. Lead can be released from lead-containing cutlery through acidic foods (fruit, wine, vegetables).
Water pipe fittings (stop cocks, fittings, angle valves, mixers) are usually made of brass or gunmetal . 3% lead is added to brass for good machinability, gunmetal contains 4–7%. Whether lead and other heavy metal ions (Cu, Zn, Ni) enter the water to a relevant extent depends on the water quality: water hardness , pH value , oxygen, salt content. In 2013 the limit value for the lead content in drinking water was reduced to 0.01 mg / L. In principle, after the water has stood in the pipe for a long time, e.g. overnight, the content of all ions that have migrated from the pipe wall can be reduced by running the water pipe for about a minute (rinsing) before it is removed for drinking water purposes.
Analytics
Classic qualitative determination of lead
Evidence by crystallization
Lead ions can be represented as lead (II) iodide in a microscopic detection reaction. The sample is dissolved in dilute hydrochloric acid and carefully evaporated until it crystallizes. The residue is taken up with a drop of water and then with a crystal of a water-soluble iodide, e.g. B. potassium iodide (KI), added. After a short time, microscopic, yellow, hexagonal leaflets of lead (II) iodide appear.
Qualitative evidence in the separation process
Since lead does not precipitate quantitatively as PbCl 2 after the addition of HCl , it can be detected both in the HCl group and in the H 2 S group. The PbCl 2 can be precipitated both by adding potassium iodide according to the above reaction as yellow PbI 2 , and with K 2 Cr 2 O 7 as yellow lead chromate, PbCrO 4 .
After H 2 S has been introduced into the hydrochloric acid sample, divalent lead precipitates in the form of black PbS. This is detected as PbI 2 or PbCrO 4 after digestion with (NH 4 ) S X and addition of 4 M HNO 3 .
Instrumental quantitative analysis of lead
A number of methods are available for trace analysis of lead and its organic derivatives. However, new or improved processes are constantly being presented in the literature, also with regard to the often necessary preconcentration. A problem that should not be underestimated is sample preparation.
Atomic Absorption Spectrometry (AAS)
Of the various AAS techniques, the quartz tube and graphite tube technology provide the best results for trace analysis of lead compounds. Often lead is converted into the volatile lead hydride, PbH 2 , with the help of NaBH 4 . This is fed into a quartz cuvette and then heated electrically to over 900 ° C. The sample is atomized and the absorbance at 283.3 nm is measured using a hollow cathode lamp. A detection limit of 4.5 ng / ml was achieved. An air-acetylene torch (F-AAS) or microwave-induced plasma (MIP-AAS) is also used for atomization in the AAS.
Atomic Emission Spectrometry (AES)
In the AES, the microwave-induced plasma (MIP-AES) and the inductively coupled argon plasma (ICP-AES) have proven themselves for atomization. The detection takes place at the characteristic wavelengths of 283.32 nm and 405.78 nm. Using the MIP-AES , a detection limit of 0.19 pg / g was determined for trimethyl lead, (CH 3 ) 3 Pb + . The ICP-AES enables a detection limit for lead in drinking water of 15.3 ng / ml.
Mass spectrometry (MS)
In nature, a total of four stable isotopes occur with different frequencies for lead. The isotope 206 Pb is often used for mass spectrometry . With the help of ICP quadrupole MS , this isotope could be determined in the urine with a detection limit of 4.2 pg / g.
Photometry
The most widely used method for photometric detection of lead is the so-called dithizone method. Dithizone is a bidentate, aromatic ligand and forms a red complex with Pb 2+ ions at pH 9-11.5, the absorbance of which is measured at 520 nm (ε = 6.9 · 10 4 l / mol · cm). Bismuth and thallium interfere with the determination and should be quantitatively precipitated or extracted beforehand.
Voltammetry
Subtractive anodic stripping voltammetry (SASV) is ideal for the electrochemical determination of traces of lead. The actual voltammetric determination is preceded by a reductive enrichment period on a rotating Ag disk electrode. The actual determination then follows by measuring the oxidation current when scanning a potential window from −800 mV to −300 mV. The measurement is then repeated without prior enrichment and the curve obtained in this way is subtracted from the first measurement. The height of the remaining oxidation peak at −480 mV correlates with the amount of lead present. A detection limit of 50 pM lead in water was determined.
Lead compounds
Lead compounds occur in the oxidation states + II and + IV. Due to the relativistic effect , the + II oxidation state is - in contrast to the lighter homologues of group 14, such as carbon and silicon - more stable than the + IV oxidation state. The fact that the oxidation number is preferred, which is lower by 2, is found in an analogous manner in other main groups and is called the effect of the inert electron pair . Lead (IV) compounds are therefore strong oxidizing agents. In intermetallic compounds of lead ( plumbids : M x Pb y ), especially with alkali and alkaline earth metals , it also adopts negative oxidation states down to −IV. Many lead compounds are salts , but there are also organic lead compounds that have a covalent structure. As with lead metal, attempts are now being made to substitute other, non-toxic compounds for lead compounds. For example, “white lead” (basic lead (II) carbonate) has been replaced as a white pigment by titanium dioxide .
Oxides
- Lead (II) oxide PbO occurs in two modifications, as red lead and as yellow massicolite. Both modifications were previously used as pigments. It serves as a raw material for other lead compounds.
- Lead (II, IV) oxide Pb 3 O 4 , also known as red lead, is a bright red powder that was previously widely used as a pigment and anti-rust paint. In Germany, and since 2005 in Switzerland as well, it is banned as rust protection. Pb 3 O 4 is used in glass production for the preparation of lead crystal .
- Lead (IV) oxide PbO 2 is a black-brown powder that is used as an electrode material in lead accumulators and as an oxidizing agent in the chemical industry (e.g. dye production).
Sulfur compounds
- Lead (II) sulfide PbS is as galena ( galena ) the main lead mineral. It serves v. a. for the production of metallic lead.
- Lead (II) sulfate PbSO 4 also occurs naturally as anglesite and was used as a white pigment.
More lead salts
- Lead (II) acetate Pb (CH 3 COO) 2 · 3H 2 O, also called lead sugar, used to be a sugar substitute e.g. B. for sweetening wine. Due to the toxicity of lead sugar, people used to die from such poisoned wine.
- Lead (IV) acetate (Pb (CH 3 COO) 4 ) forms colorless crystal needles that smell of vinegar in moist air. With water it decomposes to lead (IV) oxide and acetic acid. It serves as a strong oxidizing agent in organic chemistry.
- White lead , basic lead carbonate 2 PbCO 3 · Pb (OH) 2 , used to be a popular white pigment; today it is mostly replaced by titanium oxide.
- Lead (II) nitrate Pb (NO 3 ) 2 is a poisonous, white powder that was used for explosives and for making matches.
- Lead (II) chloride PbCl 2 is used as the starting material for the production of lead chromate.
- Lead (II) chromate PbCrO 4 is an orange-yellow powder that used to be used as a pigment and is no longer used today because of its toxicity.
- Lead azide Pb (N 3 ) 2 is an important initial explosive .
Organic lead compounds
Organic lead compounds are almost always in the +4 oxidation state. The best-known of these is tetraethyl lead Pb (C 2 H 5 ) 4 (TEL), a poisonous liquid that was added to gasoline as an anti- knock agent. Today tetraethyl lead is only used in aviation fuel .
literature
- Gerhart Jander, Ewald Blasius: Introduction to the inorganic-chemical internship. 14th edition. S. Hirzel, Leipzig 1995, ISBN 3-7776-0672-3 .
- William H. Brock: Vieweg's History of Chemistry. Vieweg, Braunschweig 1997, ISBN 3-540-67033-5 .
- Stefan Meier: Lead in ancient times. Mining, smelting, long-distance trade. Dissertation . Zurich, University 1995.
- Raymund Gottschalk, Albrecht Baumann: Material provenance of late-Roman lead coffins in the Rheinland, Germany. In: European Journal of Mineralogy . 13, Stuttgart 2001, pp. 197-200.
- Heiko Steuer, Ulrich Zimmermann: Old mining in Germany . (= Archeology in Germany. Special issue). Konrad Theiss, Stuttgart 1993, ISBN 3-8062-1066-7 .
Web links
- Mineral Atlas: Lead (Wiki)
- MATERIAL ARCHIVE: Lead - Extensive material information and pictures
- Lead Poisoning: A Historical Perspective - Historical perspective for lead poisoning (English)
- Investigation of battery recycling processes and systems with regard to ecological and economic relevance with special consideration of the cadmium problem ( Memento of April 13, 2012 in the Internet Archive ) (2001, PDF, pp. 215–226; 1.37 MB)
- TRGS 505-Technical rules for hazardous substances-lead (PDF; 186 kB)
- Discharge of lead into the environment (PDF; 3.9 MB)
- Federal Environment Agency: Material monograph lead (1996) ( Memento from September 27, 2007 in the Internet Archive ) (PDF; 48 kB)
- Molecular cause of lead poisoning
- Lead and Human Health , Environmental Health & Toxicology, Specialized Information Services, National Library of Medicine (en.)
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (lead) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e entry on lead in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on lead at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 482.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-142-4-147. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ a b C. A. Sutherland, EF Milner, RC Kerby, H. Teindl, A. Melin, HM Bolt: Lead. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2006, doi : 10.1002 / 14356007.a15_193.pub2
- ↑ a b c d Entry on lead, powder in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ Entry on Lead in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on April 21, 2020.
- ↑ Gmelin Institute for Inorganic Chemistry and Limits: Lead Part A 1: History . Springer-Verlag, 2013, ISBN 978-3-662-11844-3 , p. 6 ( limited preview in Google Book search).
- ↑ Betina Faist : The long-distance trade of the Assyrian Empire between the 14th and 11th centuries BC (= Old Orient and Old Testament. Volume 265). Ugarit Verlag, Münster 2001, p. 45.
- ↑ a b Hans Schwerteck: lead. In: The New Pauly (DNP). Volume 2, Metzler, Stuttgart 1997, ISBN 3-476-01472-X , Sp. 707-709, here Sp. 707.
- ↑ Peter Kritzinger : A new testimony from an old friend: lead seal, lead trade and lead production in free Germania. In: Marburg contributions to ancient trade, economic and social history . Volume 35, 2017, pp. 87-107, here p. 102 (especially note 55) ( online ).
- ↑ a b c Hans Schwerteck: lead. In: The New Pauly (DNP). Volume 2, Metzler, Stuttgart 1997, ISBN 3-476-01472-X , Sp. 707-709, here Sp. 708.
- ↑ Dietwulf Baatz : Slingshots made of lead. A weapons investigation. In: Saalburg yearbook. Volume 45, 1990, pp. 59-67.
- ↑ Vitruv, De architectura 8,6,10–11 (text: Latin German ).
- ↑ BENZINBLEIGESETZ: BENZINBLEIGESETZ , accessed on March 24, 2018
- ↑ Bill Bryson: A Brief History of Almost Everything . Goldmann Verlag, 2011, ISBN 978-3-641-07924-6 , pp. 219 ( limited preview in Google Book search).
- ↑ Wolfgang Piersig: lead - metal of antiquity, the present, with a future, a material for technology, culture, art . GRIN Verlag, 2011, ISBN 978-3-656-07290-4 , pp. 8 ( limited preview in Google Book search).
- ↑ Federal Institute for Occupational Safety and Health: BAuA - Documentations - Documentation of the stakeholder workshop "Renovation of wooden windows with leaded coatings" on February 16, 2009 - Federal Institute for Occupational Safety and Health , accessed on March 24, 2018
- ↑ Technische Universität Dresden, Faculty of Environmental Sciences: Investigations into the distribution of lead-free hunting ammunition - A diffusion-theoretical consideration on the acceptance of a potential environmental innovation , doctoral thesis by Jan Engel
- ↑ Printed matter 700/17 (resolution) December 15, 2017 on the regulation on the reorganization of drinking water regulations , resolution B 4.
- ^ A b Lower Saxony State Health Office : Questions and answers about lead in drinking water , accessed on January 21, 2020
- ↑ Art. 67 Paragraph 1 of Regulation (EC) No. 1907/2006 i. V. m. with Annex XVII No. 30 to this so-called REACH regulation and with Annex VI Part 3 to Regulation (EC) No. 1272/2008 (the so-called CLP regulation ). The ban resulted from the inclusion of lead as a powder (particle size below 1 mm, index no. 082-13-00-1) or massive (index no. 082-14-00-7) in the list of substances hazardous to reproduction (here Category 1A) in accordance with Table 3 of the aforementioned Annex to the CLP Regulation and in accordance with Appendix 5 of the aforementioned Annex to the REACH Regulation by the Commission Regulation (EU) 2017/1510 of August 30, 2017 with effect from March 1, 2018 A violation of this prohibition of placing on the market z. B. by selling such lead to "private" for lead casting u. is therefore in Germany according to § 5 No. 20 Chemical Sanctions Ordinance i. V. m. Section 27 of the Chemicals Act is a criminal offense (as of July 2019).
- ↑ Hans Breuer: General and inorganic chemistry. (= dtv-Atlas Chemie. Volume 1). 9th edition. dtv, Munich 2000, ISBN 3-423-03217-0 , p. 151.
- ↑ a b List of localities for solid lead in the Mineralienatlas and Mindat
- ↑ a b Lead . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 57 kB ; accessed on January 12, 2018]).
- ↑ Webmineral - Mineral Species sorted by the element Pb (Lead) (English).
- ↑ Fraunhofer Institute: Trends in the supply and demand situation for mineral raw materials. ( Memento of March 10, 2013 in the Internet Archive ) (PDF, 350 p .; 2.1 MB).
- ↑ a b Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 396 (first edition: 1891).
- ↑ Richard V. Gaines, H. Catherine W. Skinner, Eugene E. Foord, Brian Mason , Abraham Rosenzweig: Dana's New Mineralogy . 8th edition. John Wiley & Sons, New York (et al.) 1997, ISBN 0-471-19310-0 , pp. 5 .
- ^ IMA / CNMNC List of Mineral Names; July 2019 (PDF 1.67 MB; lead (lead) see p. 106)
- ^ IMA / CNMNC List of Mineral Names; 2009 (PDF 1.8 MB, lead (lead) see p. 161).
- ↑ Webmineral - Minerals Arranged by the New Dana classification. 01/01/01 Gold group
- ↑ Image examples of platy and dendritic lead at mindat.org
- ↑ Example of a microscopic image of octahedral lead crystals at mindat.org
- ↑ United States Geological Survey.
- ↑ Statistics: Worldwide lead consumption up to 2016 | Statistics , accessed March 24, 2018
- ↑ Statistics: • Worldwide lead production until 2016 | Statistics , accessed March 24, 2018
- ↑ QSL at berzelius.de ( Memento from September 29, 2007 in the Internet Archive )
- ↑ a b Michael Binnewies: General and inorganic chemistry. Spectrum, Heidelberg 2004, ISBN 3-8274-0208-5 .
- ^ Ralph WG Wyckoff: Crystal Structures . 2nd Edition. tape 1 . John Wiley & Sons, New York, London, Sydney 1963, pp. 3 (in the appendix ).
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 35 .
- ↑ Data sheet lead (PDF) from Merck , accessed on February 23, 2010.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ P. de Marcillac, N. Coron, G. Dambier, J. Leblanc, J.-P. Moalic: Experimental detection of α-particles from the radioactive decay of natural bismuth. In: Nature . 422, 2003, pp. 876-878; doi: 10.1038 / nature01541 .
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729. Amsterdam 2003, pp. 3–128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ Neutrino hunt, lead from ancient ship protects high-tech experiment. In: Spiegel Online. March 19, 2010. Retrieved March 19, 2010.
- ↑ F. Scheiding: About the protective measures in the production of nitroglycerine. In: Angewandte Chemie . 3, 20, 1890, pp. 609-613; doi: 10.1002 / anie.18900032002 .
- ↑ RoHS: New exceptions to the requirements for the use of substances: RoHS: New exceptions to the requirements for the use of substances ( memento of March 24, 2018 in the Internet Archive ), accessed on March 24, 2018
- ↑ Too much lead in kitchen fittings. on: orf.at August 19, 2011, accessed on April 28, 2012.
- ↑ List of MAK and BAT Values 2017, Communication 53, Senate Commission for the testing of hazardous substances , Wiley-VCH Verlag, July 21, 2017, ISBN 978-3-527-81211-0 , Chapter III. Carcinogenic substances ( doi : 10.1002 / 9783527812110.ch3 , free full text) and MAK and BAT value list 2017 ( doi : 10.1002 / 9783527812110.oth , free full text).
- ↑ Katja Bauer: Toxins on the shooting ranges of elite police? on: badische-zeitung.de , June 4, 2016, (June 4, 2016)
- ↑ Sport shooters should use lead-free ammunition. In: badische-zeitung.de , Panorama. June 9, 2016. (June 11, 2016)
- ↑ F. Scheffer, P. Schachtschabel: Textbook of soil science. 13th edition. Enke Verlag, Stuttgart 1992, ISBN 3-432-84772-6 , chap. XXII 4 d.
- ↑ Environmental data (p. 31). (PDF; 4.1 MB).
- ^ Lead shot and the environment, Nabu; Aachen district association .
- ↑ Hunt with lead ammunition, SWR .
- ↑ Carvalho FM, Neto AM, Peres MF, et al: Intoxicação pelo chumbo: Zinco protoporfirina no sangue de crianças de Santo Amaro da Purificação e de Salvador , J Pediatr (Rio J). 1996; 72 (5): 295-298. doi: 10.2223 / jped.629
- ↑ Environmental data (pp. 28 and 35). (PDF; 4.1 MB).
- ↑ Heavy metals in food: Heavy metals in food , accessed on March 24, 2018
- ↑ Stiftung Warentest warns of lead in drinking water , March 19, 2010, accessed on January 2, 2013.
- ↑ Gunmetal versus brass. ( Memento of May 28, 2013 in the Internet Archive ) at: messing-sanitaer.de , accessed on February 3, 2014.
- ↑ Corrosion on metallic materials in drinking water. on: trinkwasserspezi.de , accessed on February 3, 2014.
- ↑ G. Jander, E. Blasius: Textbook of analytical and preparative inorganic chemistry. 16th edition. S. Hirzel-Verlag, Stuttgart 2005, ISBN 3-7776-1388-6 , pp. 533, 472, 540-541.
- ↑ N. Maleki, A. Safavi, Z. Ramezani: Determination of lead by hydride generation atomic absorption spectrometry (HGAAS) using a solid medium for generating hydride. In: J Anal At Spectrom . 14, 1999, pp. 1227-1230; doi: 10.1039 / A808429G .
- ↑ M. Heisterkamp, F. Adams: In situ propylation using sodium tetrapropylborate as a fast and simplified sample preparation for the speciation analysis of organolead compounds using GC-MIP-AES. In: J. Anal. At. Spectrom. 14, 1999, pp. 1307-1311; doi: 10.1039 / A901340G .
- ↑ M. Zougagh, A. Garcia de Torres, E. Alonso, J. Pavon: Automatic on line preconcentration and determination of lead in water by ICP-AES using a TS-microcolumn. In: Talanta . 62, 2004, pp. 503-510; doi: 10.1016 / j.talanta.2003.08.033 .
- ↑ Z. Chen, N. Zhang, L. Zhuo, B. Tang: Catalytic kinetic methods for photometric or fluorometric determination of heavy metal ions. In: Microchim Acta . 164, 2009, pp. 311-336; doi: 10.1007 / s00604-008-0048-8 .
- ^ A. Townsend, K. Miller, St. McLean, St. Aldous: The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS. In: J. Anal. At. Spectrom. 13, 1998, pp. 1213-1219; doi: 10.1039 / A805021J .
- ↑ R. lobinski, Z. Marczenko: Spectrochemical Trace Analysis for Metals and metalloid. Elsevier 1997, ISBN 0-444-82879-6 .
- ^ I. Oehme, OS Wolfbeis: Optical Sensors for Determination of Heavy Metal Ions. In: Microchim. Acta . 126, 1997, pp. 177-192; doi: 10.1007 / BF01242319 .
- ↑ B. Lange, ZJ Vejdelek: Photometric analysis. Verlag Chemie, Weinheim 1980.
- ↑ Y. Bonfil, E. Kirowas-Eisner: Determination of nanomolar Concentrations of lead and cadmium by anodic-stripping voltammetry at a silver electrode. In: Anal. Chim. Acta . 457, 2002, pp. 285-296; (PDF) ( Memento from January 30, 2012 in the Internet Archive )
- ^ J. Wang: Stripping Analysis at Bismuth Electrodes: A Review. In: Electroanalysis . 17, 2005, pp. 1341-1346; doi: 10.1002 / elan.200403270 .