Organic lead compounds
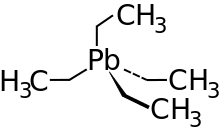
Organic lead compounds (also lead organyls, organic lead compounds or organoplumbanes) are chemical compounds of lead with organic residues. Compared to many organic compounds of other elements, they are often relatively stable and do not react with air or water under normal conditions . Organic lead compounds are poisonous. The best known of these compounds is tetraethyl lead , which was added to gasoline in large quantities as an anti-knock agent .
history
Hexaethyldiblei, the first organic lead compound, was reported by Carl Löwig in 1853 . In 1922, Thomas Midgley and TA Boyd discovered the effect of tetraethyl lead as an anti-knock agent in gasoline engines.
Extraction and presentation
There are several ways to represent organic lead compounds. Technically tetraethyl and were tetramethyl lead mainly by reaction of a lead sodium - alloy with ethyl chloride or methylene chloride produced.
Tetraalkyl lead compounds can also be prepared by reacting lead salts such as lead (II) chloride or lead (IV) acetate with Grignard compounds or organolithium compounds .
Another possibility is the reaction of a Grignard compound and an alkyl halide with a soluble lead anode in the presence of an inert cathode:
Lead (II) compounds are less stable and react with disproportionation :
The organo lead halides R 3 PbX and R 2 PbX 2 (X: halogen) can be obtained from the tetraalkyl lead compounds by reaction with halogens , hydrogen halides or thionyl chloride .
These, in turn, are the starting product for the preparation of other organic lead compounds in which the halogen is replaced by hydroxide, alkoxides , a hydrogen atom or other alkyl radicals. The corresponding alkyl lead hydrides are significantly less thermally stable than the corresponding organotin compounds and decompose quickly, sometimes explosively at temperatures above 0 ° C.
properties
In organic lead compounds, lead is almost always in the +4 oxidation state, while compounds in lower oxidation states are rare and unstable. Lead forms chains only with difficulty due to the low binding energy of lead-lead bonds .
The lead- carbon bond is relatively weak and polarizable, so that this bond can break both in radicals and in ions. It has a length of 225 pm , which is significantly greater than the length of the carbon-carbon single bond of 154 pm. The energy of the lead-carbon bond, at 206 kJ / mol, is also well below the energy of the carbon-carbon bond. This is important for use as an anti-knock agent. The lead-carbon compound is hydrolysis-stable in lead (IV) -Organylene but has the lowest thermal stability in the group, which is why the compounds decompose at 100 to 200 ° C.
If some of the alkyl radicals are replaced by other groups, the compound becomes significantly more reactive. It is so that the reactivity increases with a decreasing number of alkyl groups. Compounds of the type RPbX 3 are similarly unstable as lead (IV) chloride . In terms of their reactivities, the substituted organo lead compounds are similar to the corresponding tin compounds , but are generally more reactive. Organoblead hydrides react easily with aldehydes , ketones , organic halogen compounds and alkynes .
safety instructions
Organic lead compounds are often fat-soluble and can then easily get through the skin or the lungs into the human body and thus via the bloodstream to the liver, kidneys and muscles. In the human body, tetraalkyl compounds are rapidly converted to trialkyl compounds in the liver. Trialkyl lead compounds in particular can cross the blood-brain barrier and accumulate in the brain. Symptoms include headache, depression, sleep disorders, hallucinations and convulsions. Parkinsonism and paralysis are described as late effects. Similar to the organotin compounds , the substances that release ions of the R 3 Pb + and R 2 Pb 2+ types are particularly toxic because they block glutathione transferase or enzymes , among other things . Organic lead substances are more toxic than organic tin compounds.
use
The possible uses of organic lead compounds, for example as biocides or plastic additives, are severely limited by their toxicity. For this reason, the corresponding tin compounds are usually preferred to lead compounds in syntheses, although the latter are usually more reactive and therefore allow milder reaction conditions.
Tetramethyl and tetraethyl lead have been used in large quantities as anti-knock agents. Both compounds have high octane numbers because they can effectively scavenge radicals. Due to the widespread use, organic lead compounds accumulated in the environment, e.g. B. The content of organic lead compounds in wine increased from the 1950s to the 1970s. Due to their toxicity, the use of organic lead compounds as anti-knock agents is now banned in many countries. In the year 2000, however, 34,000 tons of lead organyls were still used as anti-knock agents.
Some other organic lead compounds such as tributyl lead acetate can be used as a wood or cotton preservative.
literature
- Christoph Elschenbroich: Organometallic chemistry. 6th edition, Teubner Wiesbaden, 2008, ISBN 978-3-8351-0167-8 , pp. 190-200.
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1028-1041.
Individual evidence
- ↑ a b c d Bernard Jousseaume: Organometallic Synthesis and Chemistry of Tin and Lead Compounds . In: Microchimica Acta . tape 109 , no. 1-4 , 1992, pp. 5–12 , doi : 10.1007 / BF01243203 (English).
- ↑ On Methplumbäthyl . In: Annals of Chemistry and Pharmacy . tape 88 , no. 3 , 1853, p. 318 , doi : 10.1002 / jlac.18530880318 .
- ^ A b Dodd S. Carr: Lead Compounds . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a15_249 .
- ^ A b Christoph Janiak, Hans-Jürgen Meyer, Dietrich Gudat, Ralf Alsfasser: Riedel Modern Inorganic Chemistry . Walter de Gruyter, January 27, 2012, ISBN 978-3-11-024901-9 , p. 619–.
- ↑ a b c d Toxicology of Working Materials by Hermann M. Bolt, Institute for Occupational Physiology at the University of Dortmund (IfaDo), 2005 (PDF file).
- ↑ a b Axel Diefenbach: Heavy metals in the environment. In: chemryb.at. Retrieved October 18, 2015 .
- ↑ a b Christoph Elschenbroich: Organometallchemie . Springer-Verlag, March 9, 2013, ISBN 978-3-322-99393-9 , p. 200–.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 983.
- ↑ Alfred Hirner, Heinz Rehage, M. Sulkowski: Environmental Geochemistry: Origin, Mobility and Analysis of Pollutants in the Pedosphere . Springer-Verlag, March 8, 2013, ISBN 978-3-642-93711-8 , p. 412.
- ↑ Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Ulfert Onken, Regina Palkovits, Albert Renken: Technische Chemie . Wiley, January 28, 2014, ISBN 978-3-527-67409-1 , pp. 687-.


![{\ displaystyle \ mathrm {2RCl + 2 \ RMgCl \ {\ xrightarrow [{}] {Pb-Anode}} \ PbR_ {4} + \ 2 \ MgCl_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c6427b9ba4cba8ca40dbdf69763c331fbdebc673)
![{\ displaystyle \ mathrm {2 \ PbX_ {2} +4 \ RMgX {\ xrightarrow [{- 4MgX_ {2}}] {}} \ 2PbR_ {2} \ longrightarrow \ PbR_ {4} + \ Pb}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/07620e8fb8b9088f3f71fcce6b647b27507d234c)

