Lead (II) chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Pb 2+ __ Cl - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lead (II) chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | PbCl 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 278.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.85 g cm −3 |
||||||||||||||||||
| Melting point |
500 ° C |
||||||||||||||||||
| boiling point |
950 ° C |
||||||||||||||||||
| solubility |
in water: 4.42 g L −1 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−359 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lead (II) chloride (PbCl 2 ) is the lead (II) salt of hydrochloric acid . It crystallizes out in white, rhombic needles or prisms .
Occurrence
Lead (II) chloride occurs naturally as a mineral cotunnite .
Extraction and presentation
Lead (II) chloride can be produced by reacting lead or lead (II) oxide with chlorine or hydrochloric acid.
Since lead (II) chloride is sparingly soluble in water, it precipitates from lead-containing aqueous solutions. This is used in the hydrochloric acid group of the cation separation process to separate lead from other ions.
properties
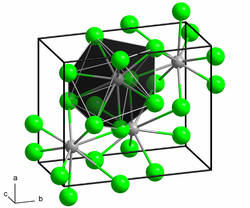
Lead (II) chloride crystallizes like lead (II) fluoride and lead (II) bromide in a spatial structure related to the uranium (III) chloride structure. Each lead atom is coordinated by a total of nine chloride atoms. As coordination polyhedra, these form distorted, triple-capped, trigonal prisms that are connected to one another via common surfaces.
While lead (II) chloride is only slightly soluble in cold, dilute hydrochloric acid, it dissolves well in concentrated hydrochloric acid with the formation of chloroplumbates. It is also soluble in ammonium chloride and nitrate solutions , as soluble complexes are formed in the process.
use
Lead (II) chloride is used in the manufacture of lead chromate and as a flux . It was also previously used to make several pigments, Pattinson's white lead (lead hydroxide chloride) and Veronese yellow (lead oxide chloride) .
Individual evidence
- ↑ a b c d Data sheet lead (II) chloride (PDF) from Merck , accessed on January 19, 2011.
- ↑ CRC Handbook of Chemistry and Physics 67th edition (1986/87)
- ↑ a b Entry on lead (II) chloride in the GESTIS substance database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry lead compounds with the exception of those named in this annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 14, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ PAETEC formula collection 2003 edition, page 116
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1013-1015.
- ↑ a b Gerhart Jander, Ewald Blasius: Introduction to the inorganic-chemical practical course: (including the quantitative analysis); with 61 tables . Ed .: Joachim Strähle, Rolando Rossi. 14., rework. / by Joachim Strähle and Eberhard Schweda. Hirzel, Stuttgart 1995, ISBN 3-7776-0672-3 , p. 245 .
- ↑ a b Entry on lead chloride. In: Römpp Online . Georg Thieme Verlag, accessed on May 25, 2014.




