Cotunnite
| Cotunnite | |
|---|---|
| Cotunnite with galena, found at: Chr. Levin colliery, Essen | |
| General and classification | |
| other names | |
| chemical formula | PbCl 2 |
|
Mineral class (and possibly department) |
Halides |
|
System no. to Strunz and to Dana |
3.DC.85 ( 8th edition : III / D.08) 02/09/07/01 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-dipyramidal; 2 / m 2 / m 2 / m |
| Space group | Pnam (No. 62, position 6) |
| Lattice parameters | a = 7.62 Å ; b = 9.04 Å; c = 4.53 Å |
| Formula units | Z = 4 |
| Twinning | along {120} |
| Physical Properties | |
| Mohs hardness | 2.5 |
| Density (g / cm 3 ) | measured: 5.80 (synthetic); calculated: 5.91 |
| Cleavage | completely after {010} |
| Break ; Tenacity | slightly mussel-like; easy to cut |
| colour | colorless to white, light green, light yellow |
| Line color | White |
| transparency | transparent to opaque |
| shine | Diamond shine, silky to pearly |
| Crystal optics | |
| Refractive indices |
n α = 2.199 n β = 2.217 n γ = 2.260 |
| Birefringence | δ = 0.061 |
| Optical character | biaxial positive |
| Axis angle | 2V = 67 ° (measured); 68 ° (calculated) |
Cotunnite is a rarely occurring mineral from the mineral class of halides with the chemical composition PbCl 2 and is therefore chemically lead (II) chloride .
Cotunnite crystallizes in the orthorhombic crystal system and develops prismatic, needle-like or tabular crystals up to about 2 mm in size along the a-axis [100] . Also known are scepter-shaped or skeletal crystals as well as radial, tufted, granular to coarse mineral aggregates and crusty coatings.
Etymology and history
Cotunnite was first discovered in 1825 by Teodoro Monticelli (1759-1845) and Nicola Covelli in the type locality , Vesuvius near Naples in Italy . They named the mineral after the Italian anatomy professor Domenico Cotugno (also Cotunnius , 1736–1822).
classification
Already in the outdated, but partly still in use 8th edition of the mineral classification according to Strunz , the cotunnite belonged to the mineral class of "halides" and there to the department of "oxyhalides", where together with challacolloite , fiedlerite , hephaistosite , laurionite , paralaurionite and pseudocotunnite the "Fiedlerit-Laurionit-Gruppe" with the system no. III / D.08 .
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), classifies the cotunnite in the expanded division of "oxyhalides, hydroxyhalides and related double halides". However, this is subdivided according to the predominant metals in the compound, so that the mineral can be found according to its composition in the subsection "With Pb (As, Sb, Bi) without Cu", where it is the only member of the unnamed group 3.DC .85 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the cotunnite to the class and there in the division of the same name of "halides". Here he is the namesake of the " Cotunnit group " with the system no. 09.02.07 and the other members Hydrophilit and Coccinit can be found in the subsection "Anhydrous and hydrous halides with the formula AX 2 ".
Education and Locations
Cotunnite can form in several ways. In volcanoes it is a sublimation product . In addition, it forms through weathering of galena , lead-containing archaeological finds or slag under salty conditions. It is associated with galena, depending on the locality, Cerussite , Anglesite and matlockite (Caracoles in Chile) or Tenorite , Ponomarevit , Sofiit , Burnsit , Ilinskit , Georgbokit , Chloromenit , halite , [sylvite] and Gold (volcanic Tolbachnik, Russia).
In addition to the type locality, a number of other sites are known. These include the Hohe Tauern in Austria , Caracoles and Challacollo in Chile , Sainte-Marie-aux-Mines in France , Röhrnbach , Richelsdorf , Essen , Recklinghausen and Helbra in Germany , Laurion in Greece , Tuscany in Italy , and the Tolbatschik in Russia , Wanlockhead , Leadhills and other localities in Great Britain and the US states of Arizona , Massachusetts and Utah .
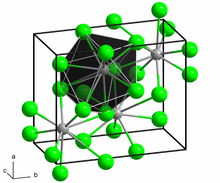
Crystal structure
Cotunnite crystallizes in the orthorhombic crystal system in the space group Pnam (space group no. 62, position 6) with the lattice parameters a = 7.62 Å , b = 9.04 Å and c = 4.53 Å as well as four formula units per unit cell .
Cotunnit gives the cotunnite structure its name, in which, in addition to lead (II) chloride, other salts such as barium chloride , calcium hydride or lead (II) bromide crystallize.
See also
literature
- T. Monticelli, N. Covelli: Cotunnia (Piombo muriato) . In: Prodromo della Mineralogia Vesuviana . tape 1 , 1825, p. 47–52 ( rruff.info [PDF; 199 kB ; accessed on June 18, 2018]).
- YZ Nozik, LE Fykin, LA Muradyan: Crystal structure of contunnite PbCl2 determined more precisely by application of the neutron diffraction method . In: Soviet Physics - Crystallography . tape 21 , 1976, p. 38-40 .
- Martine Lumbreras, J. Schram, Joop Schoonman, EJL Schouler: Electrical conductivity of mixed lead halides PbCl 2x Br 2 (1 - x) . In: Solid State Ionics . tape 28–30 , no. 2 , 1988, p. 1305-1309 , doi : 10.1016 / 0167-2738 (88) 90376-1 .
Web links
- Mineral Atlas: Cotunnite
- RRUFF Database-of-Raman-spectroscopy - Cotunnite (English)
- American-Mineralogist-Crystal-Structure-Database - Cotunnite (English)
Individual evidence
- ↑ a b c d Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 179 .
- ↑ Webmineral - Cotunnite (English)
- ↑ a b c d e Cotunnite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 70 kB ; accessed on June 18, 2018]).
- ↑ a b c d e Mindat - Cotunnite (English)
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 .
- ↑ Biographi of Teodoro Monticelli at www.minerbook.it
- ↑ Marco E. Ciriotti, Lorenza Fascio, Marco Pasero: Italian Type Minerals . 1st edition. Edizioni Plus - Università di Pisa, Pisa 2009, ISBN 978-88-8492-592-3 , p. 92 .