Barium chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Ba 2+ __ Cl - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Barium chloride | ||||||||||||||||||
| other names |
Chlorbarium (obsolete) |
||||||||||||||||||
| Ratio formula | BaCl 2 | ||||||||||||||||||
| Brief description |
colorless, cubic or monoclinic crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass |
|
||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.86 g cm −3 |
||||||||||||||||||
| Melting point |
963 ° C |
||||||||||||||||||
| boiling point |
1560 ° C |
||||||||||||||||||
| solubility |
good in water (375 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.5 mg m −3 (Ba) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−859.8 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Barium chloride is a colorless, crystalline chemical compound and a chloride of barium . Barium chloride is often present as a dihydrate (BaCl 2 · 2 H 2 O).
Extraction and presentation
Barium chloride can be represented according to all salt formation reactions :
- Barium reacts with chlorine to form barium chloride.
- Barium hydroxide reacts with hydrochloric acid to form barium chloride and water.
- Barium oxide reacts with hydrochloric acid to form barium chloride and water.
Commercially, barium chloride is synthesized by the reaction of barium sulfide with hydrochloric acid to form hydrogen sulfide acid:
The reaction of barium carbonate with hydrochloric acid also produces barium chloride with the simultaneous formation of water and carbon dioxide :
properties
Physical Properties
Barium chloride is a colorless, crystalline powder with a melting point of 963 ° C. The melt boils at 1560 ° C. Like barium and all its salts, barium chloride has a green flame color , it is readily soluble in water and, like all soluble barium compounds, is toxic.
Barium chloride usually occurs in connection with two molecules of crystal water as barium chloride dihydrate. Anhydrous barium chloride is obtained when the water is removed from the barium chloride dihydrate by means of heat ( dehydration ). Barium chloride dihydrate is also a white, crystalline powder.
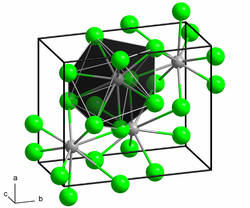
Anhydrous barium chloride crystallizes orthorhombically , space group Pnma (space group no. 62) , with the lattice parameters a = 7.865 Å , b = 4.731 Å and c = 9.421. The dihydrate crystallizes monoclinically , space group P 2 1 / n (No. 14, position 2) , with the lattice parameters a = 6.722 Å, b = 10.91 Å, c = 7.132 Å and β = 91.10 °.
Chemical properties
Reaction with sulfate ions:
- Magnesium sulfate reacts with barium chloride to form barium sulfate and magnesium chloride.
Reaction with potassium chromate :
- Barium chloride reacts with potassium chromate to form barium chromate and potassium chloride.
use
Barium chloride is used as an indicator for sulfate ions, since barium sulfate precipitates out as a white solid when it reacts with sulfate ions (see reactions) . This precipitation reaction can also be used to purify sodium chloride from sulfates.
In addition, barium is used for hardening of steel , in the pyrotechnics due to its green flame coloration and for the preparation of dyes barium sulfate (see reactions), and barium (see reactions) used.
The Red Army used during the Second World War greenish glowing barium chloride tracer bullets to the shooter to facilitate the goals, while the Wehrmacht yellowish glowing phosphorus used -Geschosse. These projectiles were used in the tanks' LMGs and MGs . These projectiles also helped other soldiers as they saw where the shooter was aiming.
toxicity
Barium chloride is slightly hazardous to water. Barium chloride is toxic by inhalation and if swallowed. In the event of an accident or if you feel unwell due to this substance, a doctor must be consulted immediately. Barium chloride must be kept locked up and out of the reach of children. In the past, barium chloride was used as a rat poison .
Barium inactivates the passive potassium channels in the membrane of the muscle cells . Potassium can no longer leave the muscle cells. Since the sodium-potassium-ATPase pumps potassium into the cells undiminished, the potassium level in the blood drops . The symptoms are hypermotility of the gastrointestinal tract , failure of the muscle reflexes ( areflexia ), flaccid muscle paralysis and respiratory paralysis . The blood test shows severe hypokalaemia .
As a first aid measure, the intake of sodium or potassium sulphate solution is recommended, as sulphate ions precipitate the barium ions and thereby form insoluble and thus non-toxic barium sulphate. In the hospital, barium can be removed by dialysis .
Individual evidence
- ↑ a b c d Entry on barium chloride. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ↑ a b c d e data sheet barium chloride (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b Entry on barium chloride in the GESTIS substance database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ Entry on Barium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ PAETEC formula collection 2003 edition, page 116
- ↑ EB Brackett, TE Brackett, RL Sass: The crystal structures of barium chloride, barium bromide and barium iodide. In: Journal of Physical Chemistry , 67, 1963, pp. 2132-2135, doi: 10.1021 / j100804a038 .
- ^ VM Padmanabhan, WR Busing, HA Levy: Barium chloride dihydrate by neutron diffraction. In: Acta Crystallographica , B34, 1978, pp. 2290-2292, doi: 10.1107 / S056774087800792X .
- ↑ C. Schmuck, B. Engels, T. Schirmeister, R. Fink: Chemistry for medical professionals . Pearson Studium, Hallbergmoos 2008, ISBN 978-3-8273-7286-4 .
- ↑ Yu-Jen Su, et al .: An Industrial Worker Hospitalized With Paralysis After an Aerosolized Chemical Exposure . In: American Journal of Kidney Diseases . 56, No. 3, 2010, pp. A38-A41. doi : 10.1053 / j.ajkd.2010.02.004 . Retrieved September 22, 2010.







