Barium iodide
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Ba 2+ __ I - | ||||||||||
| General | ||||||||||
| Surname | Barium iodide | |||||||||
| other names |
|
|||||||||
| Ratio formula | BaI 2 | |||||||||
| Brief description |
colorless and odorless crystals, hygroscopic |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
740 ° C |
|||||||||
| boiling point |
decomposition |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
−602.1 kJ / mol |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Barium iodide is the barium salt of hydriodic acid .
Manufacturing
Barium iodide can be prepared by reacting barium carbonate with hydrogen iodide .
It can also be made from barium hydroxide and iodine in the presence of a reducing agent, e.g. B. phosphorus or sulphurous acid can be produced.
It is also possible to produce it by reacting barium hydride with ammonium iodide in pyridine .
properties
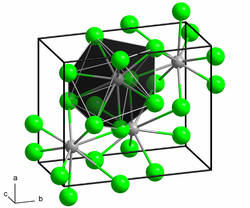
Barium iodide crystallizes in the PbCl 2 type ( Pnma , a = 892.2 (8) pm, b = 530.4 (4) pm, c = 1069.5 (8) pm and Z = 4) in which every barium cation of nine Iodide anions is surrounded. Barium iodide forms hygroscopic crystals and often occurs as the dihydrate BaI 2 · 2 H 2 O. The dihydrate gives off its water of crystallization at 150 ° C. It is very soluble in water, the solubility increases with increasing temperature. When sulfate ions are added, a white precipitate of barium sulfate separates out of the aqueous solution .
use
Barium iodide is used in homeopathy under the names Barium iodatum and Baryta iodata .
Individual evidence
- ↑ a b E. Nuremberg, P. Surmann: "Methods". ( limited preview in Google Book search)
- ↑ Data sheet barium iodide from AlfaAesar, accessed on June 2, 2010 ( PDF )(JavaScript required) .
- ↑ a b c data sheet Barium iodide, anhydrous (PDF) from Strem, accessed on December 25, 2012.
- ↑ Data sheet barium iodide from Sigma-Aldrich , accessed on June 2, 2010 ( PDF ).
- ↑ a b c Dale L. Perry, Sidney L. Phillips: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , p. 223 ( limited preview in Google book search).
- ↑ Data sheet barium iodide from Acros, accessed on June 2, 2010.
- ↑ a b c d e R. Abegg, F. Auerbach: Handbuch der inorganic Chemie . Vol. 2, Verlag S. Hirzel, 1908. P. 256ff. ( Full text ).
- ↑ a b Entry on barium iodide in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the indicated labeling it falls under the group entry barium salts, with the exception of barium sulphate, salts of 1-azo-2-hydroxynaphthalenyl aryl sulphonic acid, and of salts specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-6.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 923.
- ↑ EB Brackett, Th. E. Brackett, RL Sass, J. Phys. Chem. 1963 , 67 , 2132-2135. doi: 10.1021 / j100804a038




