Sulfates
Sulphates are salts or esters of sulfuric acid . The salts contain the sulfate anion [SO 4 ] 2− or the hydrogen sulfate anion [HSO 4 ] - . The esters of sulfuric acid have the general formula R − O − SO 2 −O − R ', where R and / or R' are organic radicals.
nomenclature
Primary and Secondary Sulphates
The salts of the dibasic acid sulfuric acid (H 2 SO 4 ) can be divided into sulfates and hydrogen sulfates (also known as primary and secondary sulfates ). For monovalent cations M I , the sum formulas M I HSO 4 and M I 2 SO 4 apply :
| Hydrogen sulfates (primary sulfates) | secondary sulfates | |
|---|---|---|
| Synonyms |
acid sulfates hydrogen sulfates bisulfates |
neutral sulfates normal sulfates |
| Examples |
Sodium hydrogen sulfate , NaHSO 4 Calcium hydrogen sulfate , Ca (HSO 4 ) 2 |
Sodium sulfate , Na 2 SO 4 calcium sulfate , CaSO 4 |
Alums and Vitriols
Alums are double salts of monovalent and trivalent cations with the general formula M I M III (SO 4 ) 2 · 12 H 2 O, the main representative of the group of alums is potassium aluminum sulfate (potassium alum). Vitriols, on the other hand, are the sulfates of bivalent subgroup metals containing water of crystallization ( copper vitriol , iron vitriol, etc.).
Sulfate complexes
Sulphate groups can appear as ligands in complexes . In this case, the ligands are referred to as [Tetraoxosulfato (−2)] or [Sulfato (−2)]. According to recommendations on nomenclature, sulphite groups (SO 3 ) 2− also have the suffix -sulphate and are referred to as trioxosulphate or [trioxosulphate (IV)].
Sulfuric acid ester
Sulfuric acid esters are sometimes also referred to as sulfates , as the names often end with -sulfate. Simple esters like dimethyl sulfate are powerful alkylating agents. Esters with longer hydrocarbon residues and sulfonic acid salts are usually surfactants . These esters also include the fatty alcohol sulfates , which are important in terms of application .
-
Mono ester; Example: sodium lauryl sulfate , a surfactant that has been used in shampoos .

-
Di ester; Example: dimethyl sulfate , a reactant that is used in the laboratory and in technology for methylation .

properties
General
Most sulfates are soluble in water . Exceptions are the little or sparingly soluble sulfates of the alkaline earth metals calcium sulfate , strontium sulfate , barium sulfate and radium sulfate as well as lead (II) sulfate . Radium sulfate is the most difficultly soluble sulfate known.
The alkali and alkaline earth sulfates in particular are extremely thermally stable. Sulphates of trivalent metal cations decompose in the heat to the corresponding oxides and sulfur trioxide :
Hydrogen sulfates are known as salts of the alkali metals . They are soluble in water. When these salts are heated, disulphates , salts of disulphuric acid, are formed .
Anions and pH
Sulfuric acid is a strong biprotonic acid. In a one-molar, aqueous solution of the acid there are almost no H 2 SO 4 molecules, but essentially HSO 4 - ions. Only about 1% of the HSO 4 - ions deprotonate to SO 4 2− . The hydrogen sulfate anion (HSO 4 - ) can act as both an acid and a base , so it is amphoteric . The pK S value of the hydrogen sulfate ion is 1.89.
If a hydrogen sulfate salt is dissolved in water, a mixture of hydrogen sulfate and sulfate ions is formed in an equilibrium reaction. Therefore, one can hydrogen sulphates as a medium strong acid used, the acid strength is considerably higher than that of acetic acid (pK S = 4.76). Due to these properties, hydrogen sulfates can also be used in buffer solutions . The buffer area is in the strongly acidic area. Because of their acidic reaction in water, they are used in toilet cleaners , for example .
| Equilibrium reactions | Equilibrium constant at 25 ° C |

|
|
|---|---|---|---|
| (1) | |||
| (2) |
Occurrence

Many metal sulfates occur naturally in the form of minerals. By far the most common is calcium sulfate (CaSO 4 ), which occurs in several mineral varieties . With water of crystallization , it is called gypsum (CaSO 4 · 2 H 2 O) or bassanite (CaSO 4 · ½ H 2 O), without anhydrite . The pure white and extremely fine-grained plaster variant is called alabaster , transparent plaster monocrystals form the Marienglas . The most important gypsum and anhydrite deposits in Central Europe are located in the so-called Zechstein series , mostly deep underground in northern and central Germany and Poland. At the edges of the Variscan mountain range, the Zechstein anhydrites sometimes reach the surface of the earth, where they are mined in quarries. A world-famous deposit of gypsum is located in Mexico in the caves opened up by the Naica mine . Other important sulfate minerals are barite (barite, BaSO 4 ), Celestine ( SrSO 4 ) and Anglesite ( PbSO 4 ).
A biological source of sulfates is the conversion of sulfides and sulfur-containing biomass components (e.g. proteins ) by sulfur-oxidizing bacteria .
Sulphates occur in different amounts in groundwater . For drinking water obtained from it, a limit value of 250 mg / l applies in Germany according to No. 17 of Annex 3 (to § 7 and § 14 paragraph 3) of the Drinking Water Ordinance.
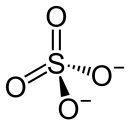
Structure of sulfate ion
The sulfate ion has a tetrahedral structure, the SO bonds are all equivalent and of the same length. The bond relationships can be described either by mesomeric boundary structures with delocalized π bonds and two negatively charged oxygen atoms or by charge separation with a doubly positively charged sulfur atom and a negative charge on each oxygen atom. It is isoelectronic with the perchlorination . The molecular orbital diagram shows how the bond and hypervalence can be explained. A doubly positively charged sulfur atom with four singly occupied atomic orbitals is assumed. These orbitals are combined with four singly occupied 2p orbitals from the singly negatively charged oxygen atoms. Four bonding and four antibonding σ-orbitals are created, of which only the bonding ones are completely filled, and thus the tetrahedral molecular structure with four localized single bonds. The unoccupied antibonding σ p * orbitals are then combined with a fully occupied p orbital each of three oxygen atoms, resulting in three binding and three antibonding π orbitals, of which only the binding orbitals are occupied. This results in three π bonds that are delocalized over the entire molecule, i.e. over the sulfur atom and all four oxygen atoms.
proof
Sulphates are detected chemically with barium chloride or barium hydroxide solution in hydrochloric acid . This creates a sparingly soluble precipitate of white barium sulfate :
- Sulphate ions and barium ions form a white, acid-insoluble precipitate of barium sulphate.
The acid is added to suppress interference, as other anions such as carbonate or sulfite with barium also form salts that are sparingly soluble in water, but that are soluble in acids.
In water analysis , titrimetric methods are also used for quantitative determination .
Examples

|
||||||||||||||||||||||||||||||||||||||||||||||||||
Further examples:
- Alums (potassium aluminum sulfate, KAl (SO 4 ) 2 12 H 2 O, and other compounds of the composition M I M III (SO 4 ) 2 12 H 2 O, such as ammonium iron (III) sulfate dodecahydrate, NH 4 Fe (SO 4 ) 2 12H 2 O, or chrome alum , KCr (SO 4 ) 2 12 H 2 O)
- Aluminite (Al 2 [(OH) 4 SO 4 ] · 7 H 2 O, a mineral)
- Ammonium iron (II) sulfate, Mohr's salt, a light green double salt made from ammonium and iron (II) sulfate
- Barite (barite, BaSO 4 , a mineral)
- Lead (II) sulfate (PbSO 4 , forms on the lead plates in car batteries through the action of sulfuric acid, white, insoluble in water, naturally occurring as angelsite )
- Calcium aluminate sulfate (Ca 6 Al 2 [(OH) 12 | (SO 4 ) 3 ] · 26 H 2 O, is used as a white pigment in paints, among other things)
- Cobalt (II) sulfate (CoSO 4 7 H 2 O, a vitriol)
- Iron (II) sulfate (FeSO 4 , green salt , contains water of crystallization, also known as iron vitriol)
- Potassium hydrogen sulfate (KHSO 4 , available as an acidic drain cleaner)
- Levosalbutamol sulfate (CAS No. 148563-16-0, the salt of a basic amine)
- Magnesium sulphate (MgSO 4 , containing water of crystallization, also known as Epsom salt and occurring in the form of kieserite as a mineral in evaporites )
- Manganese (II) sulfate (MnSO 4 , contains water of crystallization also known as manganese vitriol , pale pink)
- Sodium hydrogen sulfate (NaHSO 4 )
- Nickel sulfate (NiSO 4 , containing water of crystallization, also known as nickel vitriol , green)
- Polyhalite (evaporite mineral containing water of crystallization from potassium, magnesium and calcium sulfate, K 2 SO 4 MgSO 4 2 CaSO 4 2 H 2 O)
- Zinc sulfate (ZnSO 4 7 H 2 O, zinc vitriol)
Web links
Individual evidence
- ↑ Gmelin's Handbook of Inorganic Chemistry: Radium, System Number 31, Eighth Edition, Verlag Chemie GmbH, Berlin 1927, pages 61-62.
- ^ Gerhard Richter-Bernburg: Zechstein anhydrite - facies and genesis. Geological Yearbook, Series A. Heft 85, Federal Institute for Geosciences and Raw Materials, Hanover 1985, ISBN 978-3-510-96409-3 .
- ↑ Martin Okrusch, Siegfried Matthes: Mineralogie: An introduction to special mineralogy, petrology and deposit science. 8th edition, Springer, Berlin / Heidelberg 2009, ISBN 978-3-540-78200-1 , p. 107 ff.





![K _ {{a1}} = {\ mathrm {{\ frac {[HSO_ {4} ^ {{-}}] [H_ {3} O ^ {+}]} {[H_ {2} SO_ {4}] }}}} \ simeq 1 \ times 10 ^ {{3}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/857ea7f76c0cec67ec123404fa513cc666fbccc1)

![K _ {{a2}} = {\ mathrm {{\ frac {[SO_ {4} ^ {{2 -}}] [H_ {3} O ^ {+}]} {[HSO_ {4} ^ {{- }}]}}}} \ simeq 1 {,} 3 \ times 10 ^ {{- 2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a2e13c8d00dfb814c37167eb40729a0e288fc05)










