Aluminum sulfate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
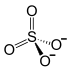
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Aluminum sulfate | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | Al 2 (SO 4 ) 3 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 342.13 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
2.71 g cm −3 |
|||||||||||||||||||||
| Melting point |
770 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
easy in water (360 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
6 mg m −3 (Al) |
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−3442 kJ mol −1 |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aluminum sulfate , empirical formula Al 2 (SO 4 ) 3 , is a chemical compound of aluminum from the group of sulfates . It forms a colorless powder with a density of 2.71 g / cm 3 .
Occurrence
In nature, for example, aluminum sulfate occurs in the mineral alunogen . It can also be obtained from naturally occurring alums .
synthesis
The hydrate of aluminum sulfate can be obtained by dissolving pure aluminum oxide or aluminum hydroxide in concentrated sulfuric acid .
Responsiveness
With an acidic reaction, aluminum sulfate dissolves in water and crystallizes out as monoclinic Al 2 (SO 4 ) 3 · 18 H 2 O at room temperature . From a temperature of 340 ° C, the salt is completely dehydrated and above 770 ° C it breaks down into aluminum oxide and sulfur trioxide .
Aluminum sulfate forms double salts with the sulfates of monovalent metals according to the following formula (alums):
use
Aluminum sulfate has the following uses:
- Retention aids and for sizing in paper manufacture
- Flocculants in drinking water treatment
- Mordants in dyeing
- Seed dressing
- Part of foam extinguishing agents
- Flame retardants
- Insulating salt (on fresh cement or lime substrates, against water and nicotine stains) in the renovation
- Ingredient in plant strengtheners and protection agents and for the blue coloration of hydrangeas
- Firming agent or stabilizer in food, approved as food additive E 520 in the EU .
Accidents and health hazards
In an incident with aluminum sulphate in drinking water at Camelford , Cornwall, England, in July 1988, a number of people drank drinking water with higher concentrations of aluminum sulphate. The permissible limit values were exceeded 5000 times. After the accident, numerous residents complained of ulcers, rashes and memory problems, among other things. A death in 2006 intensified a debate about the long-term effects of the accident. Studies of the long-term health effects following this incident are still not fully completed, but post-mortem studies found particularly high levels of aluminum in the victims' brains and further studies have been commissioned to see if there is an association with cerebral amyloid angiopathy .
In 2013, aluminum sulfate was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of aluminum sulphate were concerns about consumer use , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use, as well as the dangers arising from a possible assignment to the group of CMR substances and the possible risk sensitizing properties. The re-evaluation has been running since 2015 and is carried out by France . In order to be able to reach a final assessment, further information was requested.
Name alum
In a chemically incorrect manner, papermakers refer to aluminum sulfate as alum.
Individual evidence
- ↑ Entry on E 520: Aluminum sulphate in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on ALUMINUM SULPHATES in the CosIng database of the EU Commission, accessed on February 13, 2020.
- ↑ a b c d Entry on aluminum sulfate. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b c d Entry on aluminum sulfate in the GESTIS material database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Data sheet aluminum sulfate (anhydrous) from AlfaAesar, accessed on January 31, 2010 ( PDF )(JavaScript required) .
- ↑ PAETEC Formula Collection Edition 2003, p. 116.
- ↑ Article on the use of aluminum sulfate as a flame retardant in insulation made from renewable raw materials on EnBauSa.de; accessed in Sept. 2016
- ↑ Market overview - Insulation materials made from renewable raw materials from the specialist agency for renewable raw materials, see table on p. 29; accessed in September 2016
- ↑ Federal Office for Consumer Protection and Food Safety: Declaration of active ingredients that are contained as co-formulants in plant protection products. ( Memento of December 8, 2015 in the Internet Archive ) (As of December 1, 2009).
- ↑ Poisoned: The Camelford scandal. The Independent , April 16, 2006, accessed March 30, 2011 .
- ^ Nigel Hawkes: Alzheimers linked to aluminum pollution in tap water . In: The Times , April 20, 2006. Retrieved April 7, 2010.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Aluminum sulphate , accessed on March 26, 2019.
- ↑ Taschenbuch der Papiertechnik , Jürgen Blechschmidt, Carl Hanser Verlag GmbH & Co. KG, 2010 e ISBN 978-3-446-42322-0 Print ISBN 978-3-446-41967-4 https://doi.org/10.3139/ 9783446423220

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)



