Sodium hydrogen sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
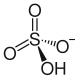
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium hydrogen sulfate | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | NaHSO 4 | ||||||||||||||||||
| Brief description |
white, crystalline, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.06 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density | |||||||||||||||||||
| Melting point |
Decomposition from 315 ° C |
||||||||||||||||||
| solubility |
very light in water (1080 g l −1 at 20 ° C, monohydrate) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sodium hydrogen sulfate (NaHSO 4 ) is an acidic sodium salt of sulfuric acid . It contains the hydrogen sulfate ion as an anion . It is also known as primary or acidic sodium sulfate ; an older name is sodium bisulfate .
The white, crystalline solid dissolves easily in water to form an acidic solution:
If the acidic sodium hydrogen sulfate is heated , it converts into sodium disulfate with the release of water ( dehydration ) :
With further heating, the sodium disulfate breaks down into sodium sulfate and sulfur trioxide :
Manufacturing
- Sodium hydrogen sulfate is synthesized by the action of moderately warm, concentrated sulfuric acid on sodium chloride . The gas hydrogen chloride is produced as a by-product :
- Drip concentrated sulfuric acid onto sodium hydroxide until there is an excess of sulfuric acid. The water escapes through the heat. It remains the desired mono hydrate back, if it is not too hot.
- Drop sulfuric acid onto sodium carbonate or sodium hydrogen carbonate until no more gas escapes. The resulting water must be removed in the desiccator . In most cases, evaporation of the solution also produces sodium disulphate. This can be moistened with water and then dried in the desiccator.
In Europe, Grillo-Werke AG produces as the largest supplier at the Höchst industrial park in Frankfurt am Main .
Applications
Sodium hydrogen sulfate is used to lower the pH in swimming pools when the water has become too alkaline.
It is the main component of acidic cleaners for household and industry.
In food technology it serves as a firming agent , acidity regulator and carrier substance . Sodium hydrogen sulphate and sodium sulphate are generally approved in the EU as food additives with the number E 514 without maximum quantity restriction ( quantum satis ) for foodstuffs.
It is also used as a litter treatment in chicken breeding.
Left: sample of sodium hydrogen sulfate. The material is very hygroscopic , so that, as can be seen in the picture, lumps are formed that are difficult to break up again.
Individual evidence
- ↑ Entry on SODIUM BISULFATE in the CosIng database of the EU Commission, accessed on April 17, 2020.
- ↑ Entry on E 500 (ii): Sodium hydrogenate carbonate in the European database for food additives, accessed on August 11, 2020.
- ↑ a b c d e f g Entry on sodium hydrogen sulfate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on sodium hydrogen sulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ SODIUM DISULFATE |. Retrieved on October 21, 2018 (German).
- ↑ Zusatzstoffe-online.de







