Ammonium iron (III) sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
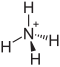 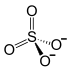
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ammonium iron (III) sulfate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
colorless solid, often pale purple due to traces of manganese |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.71 g cm −3 |
||||||||||||||||||
| Melting point |
39-41 ° C |
||||||||||||||||||
| solubility |
very good in water (1240 g l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ammonium iron (III) sulfate , is a salt consisting of iron , ammonium and sulfate ions . It has the formula NH 4 Fe (SO 4 ) 2 .
properties
The ammonium iron (III) sulfate dodecahydrate, which belongs to the group of alums, forms water-soluble, pale purple cubic crystals with a density of 1.71 g / cm 3 and a molar mass of 482.19 g / mol. The melting point is between 39 and 41 ° C. When heated from 230 ° C, it changes into the anhydrous form NH 4 Fe (SO 4 ) 2 with a molar mass of 266.00. In solution , the Berlin blue is formed with potassium hexacyanoferrate (II) , the yellow blood liquor salt .
use
Ammonium iron (III) sulfate dodecahydrate is used as an indicator for titrations according to Volhard . It is also used as a stain in the textile industry and as an astringent in medicine .
Individual evidence
- ↑ a b Entry on ammonium iron (III) sulfate. In: Römpp Online . Georg Thieme Verlag, accessed on June 27, 2014.
- ↑ a b c d data sheet ammonium iron (III) sulfate from AlfaAesar, accessed on February 1, 2012 ( PDF )(JavaScript required) .

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)

