Buffer (chemistry)
A buffer is a mixture of substances whose pH value (concentration of oxonium ions ) changes much less when an acid or a base is added than would be the case in an unbuffered system . The effect of the buffer is based on the conversion of the oxonium ions (H 3 O + ) or the hydroxide ions (OH - ) supplied by the acid or base to weak acids or bases, which themselves only slightly lead to the formation of H 3 O + or OH - ions tend.
Buffers are the aqueous buffer solutions specifically produced in chemistry . More complex buffer systems are found in body fluids such as blood or in groundwater that interacts with humus , for example .
Chemical basics
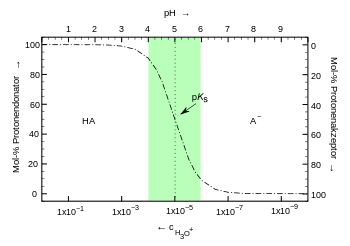
Buffer solutions contain a mixture of a weak acid and its conjugate or corresponding base (or the respective salt) or a weak base and its conjugate or corresponding acid . Also ampholytes (bifunctional molecules) may serve as a buffer. The factor determining the pH value is the ratio or the protolysis equilibrium of the buffer pair.
The following applies to the acid-base balance of an acid HA:
According to the law of mass action, the denominator would also include the concentration of the water. However, since it is very large compared to the ion concentrations of 55.6 mol / l, this can be regarded as constant and is, by definition, included in the dissociation constant K s (the acid constant ).
Forming gives:
If we form the negative decadic logarithm from this, we get:
This matches with:
respectively:
- Henderson-Hasselbalch equation (buffer equation )
With this equation, - it is valid under the approximation that the activities of the substances their concentrations correspond in solution - can be at a known pK s value for a given pH, the concentration ratio of acid and base determined. The higher the concentrations, the less the effect of adding acids or bases. The amount of strong base (or acid) that can be absorbed by a buffer solution without a significant change in pH is expressed by the buffer capacity .
Examples of buffer solutions are the acetic acid / acetate buffer or the ammonium buffer made from ammonium ions and ammonia .
Types of buffer systems
The carbonate buffer (a mixture of carbonic acid and hydrogen carbonates ) regulates the CO 2 concentration between the atmosphere, oceans and the biosphere. It is also the main part of the blood buffer . This keeps the pH of the blood between 7.35 and 7.45 and balances out the fluctuations caused by the metabolism . A pH value below 7.35 is called acidosis , above 7.45 it is called alkalosis . Death occurs at pH values below 6.8 or above 8.0.
When considering a buffer system, a distinction must be made between closed and open buffer systems. In the case of a closed buffer system (e.g. acetic acid / acetate buffer ), the protons (H + ) or hydroxide ions (OH - ) that arise during a chemical reaction are captured by the buffer substance. They react to the corresponding corresponding or conjugated acid or base of the buffer and therefore remain in the solution. With an open buffer system (e.g. the hydrogen carbonate / CO 2 buffer system in the lungs), the system is in communication with the environment. It is able to maintain the appropriate pH value by releasing a component into the environment, e.g. B. by exhaling CO 2 .
meaning
Buffers play an important role in technical chemistry, for example in electroplating or in analog photography , as well as in analytics .
Buffer systems also play an important role in soil science ; see buffer area (soil science) .
Significance in the life sciences : buffers are essential for many animals and not least for the human organism. The human blood plasma and many enzymes depend on a constant pH value. Without a buffer, even the smallest amounts of acid - e.g. B. lactic acid from the energy metabolism - are sufficient to paralyze the organism because various proteins would denature and thus become unusable.
Examples
- Barbital acetate buffer according to Michaelis (pH 2.6 to 9.2)
- Acetic acid acetate buffer (pH 3.7 to 5.7)
- Ringer's solution
-
Good buffers , including e.g. B.
- HEPES : 4- (2-Hydroxyethyl) -1-piperazineethanesulfonic acid (pH 6.8 to 8.2)
- HEPPS : 4- (2-hydroxyethyl) -piperazine-1-propanesulfonic acid (pH 7.3 to 8.7)
- MES: 2- (N-Morpholino) ethanesulfonic acid (pH 5.2 to 6.7)
- Carbonic acid silicate buffer (pH 5.0 to 6.2; slightly acidic)
- Phosphate buffer according to Sørensen (pH 5.4 to 8.0)
- McIlvaine phosphate-citrate buffer
- Carbonic acid-bicarbonate system (pH 6.2 to 8.6; neutral)
- TRIS : tris (hydroxymethyl) aminomethane (pH 7.2 to 9.0)
- Ammonia buffer: NH 3 + H 2 O + NH 4 Cl (pH 8.2 to 10.2)
- Citric acid or citrate buffer
- Bouin's solution
- Electrophoresis buffer




