Ferric sulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
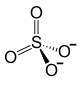
|
||||||||||
| General | ||||||||||
| Surname | Ferric sulfate | |||||||||
| Molecular formula | Fe 2 (SO 4 ) 3 | |||||||||
| Brief description |
colorless, odorless, hygroscopic, moisture- and light-sensitive powder |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 399.88 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
|
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Iron (III) sulfate is a chemical compound from the group of sulfates .
Occurrence
Iron (III) sulfate is a waste product of the chemical industry. It also occurs in the form of the ( hydrated ) minerals containing water of crystallization, coquimbit ( nonahydrate ) and quenstedtite ( decahydrate ).
Extraction and presentation
Iron (II) sulphate monohydrate FeSO 4 · H 2 O decomposes when heated above about 400 ° C to basic iron (III) sulphate and sulfur dioxide . The decomposition of iron (II) sulphate heptahydrate FeSO 4 · 7 H 2 O in air also produces it .
On an industrial scale, iron (III) sulfate is obtained by adding sulfuric acid and an oxidizing agent (e.g. nitric acid or hydrogen peroxide ) to a hot iron (II) sulfate solution.
It is also produced as an intermediate product in the Copperas process.
properties
Iron (III) sulfate dissolves in water with strong hydrolysis with a yellow-brown color (formation of, among other things, [Fe (OH) (H 2 O) 5 ] 2+ ). When aqueous solutions are boiled, basic sulfates accordingly precipitate. At low pH values it can crystallize out in the form of almost colorless hydrates (n = 3, 6, 7, 9, 10, 12). With alkali or ammonium sulfate it forms iron alum , e.g. E.g .: ammonium iron (III) sulfate (NH 4 ) Fe (SO 4 ) 2 · 12 H 2 O, which is used as an indicator in argentometry .
Iron (III) sulphate cannot be thermally stressed and splits off SO 3 when heated .
use
Iron (III) sulphate is used in large sewage treatment plants for deodorization and for the precipitation of phosphate (e.g. in drinking water treatment and industrial water disposal) and in the iron and steel industry as a pickling agent (e.g. for aluminum and steel). A hemostatic and astringent effect is known in medicine .
safety instructions
Iron (III) sulphate was included in the EU's ongoing action plan ( CoRAP ) in 2015 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of iron (III) sulphate were concerns about consumer use , exposure of workers , high (aggregated) tonnage and widespread use, as well as the possible risk of sensitizing properties. The reassessment was supposed to be carried out by Lithuania , but the reassessment of the substance was withdrawn in 2019 because the risks were assessed as low.
Individual evidence
- ↑ a b c d e f g Entry on iron (III) sulphate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-69.
- ↑ MSDS from MartinBaker. ( Memento of the original from July 9, 2008 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Richard P. Pohanish: HazMat Data For First Response, Transportation, Storage, and Security . John Wiley & Sons, 2005, ISBN 0-471-72610-9 , pp. 549 ( limited preview in Google Book search).
- ↑ a b Holleman / Wiberg: Volume 2, subgroup elements, lanthanoids, actinides, transactinides Volume 2: subgroup elements, lanthanoids, actinides, transactinides, appendices . Walter de Gruyter GmbH & Co KG, 2016, ISBN 978-3-11-049590-4 , p. 1958 ( limited preview in Google Book Search).
- ^ Rochelle M. Cornell, Udo Schwertmann: The Iron Oxides Structure, Properties, Reactions, Occurrences and Uses . John Wiley & Sons, 2003, ISBN 3-527-30274-3 , pp. 526 ( limited preview in Google Book search).
- ↑ Peter Paetzold: Chemistry an introduction . Walter de Gruyter, 2009, ISBN 978-3-11-020268-7 , pp. 856 ( limited preview in Google Book Search).
- ↑ Patent specification: Process for the production of an aqueous iron (III) sulfate solution and its use as a water treatment reagent
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): diiron tris (sulphate) , accessed on March 26, 2019.
- ↑ ECHA: Withdrawal , March 19, 2019.

![\ mathrm {\ \! \ \ Biggr] _2} \ mathrm {\ \ Biggl [}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bafeab07e1d5bb8bb77e0767e55563945c55cd72)
![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)





