Ammonium sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
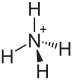 
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium sulfate | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | (NH 4 ) 2 SO 4 | |||||||||||||||
| Brief description |
odorless and colorless crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 132.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.77 g cm −3 |
|||||||||||||||
| Melting point |
> 235 ° C |
|||||||||||||||
| solubility |
easily soluble in water:
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
|
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−1180 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium sulfate (E 517) is a salt of ammonia and sulfuric acid . A related salt is ammonium hydrogen sulfate .
Occurrence
In nature, ammonium sulfate is known as a rare mineral under the name of mascagnin.
properties
Ammonium sulfate is not very hygroscopic , aqueous solutions are weakly acidic. Like many ammonium salts, it can react with sodium nitrite or potassium nitrite at room temperature to form flames or even to explode.
Extraction and presentation
Ammonium sulfate is made by introducing ammonia into 80% sulfuric acid
or made by reacting ammonia , carbon dioxide and water with gypsum .
use
Ammonium sulfate is an important fertilizer additive . In biological sewage treatment plants it is used as a component of nutrient mixtures for the microorganism cultures .
In the chemical industry, it is used, among other things, as a precipitant for protein , as a flotation agent and for the production of fire extinguishing powder and flame retardants . The leather industry uses it for staining, the paper industry for flame-retardant papers .
In biochemistry , ammonium sulfate is the salting out of proteins ( ammonium sulfate precipitation ) was used. In addition, cells and tissue are preserved in a saturated ammonium sulfate solution to protect the RNA from degradation.
Ammonium sulfate is also added to foods as a carrier for other additives. In the EU , as a food additive with the number E 517, it is only approved as a carrier or carrier solvent.
In winemaking , ammonium sulphate is used as a component of nutrient salt mixtures for yeasts , above all to promote cell reproduction during fermentation.
Web links
Individual evidence
- ↑ Entry on E 517: Ammonium sulphate in the European database for food additives, accessed on August 11, 2020.
- ↑ Entry on AMMONIUM SULFATES in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b c d e f g h i j Entry on ammonium sulphate in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ Robert K. Scopes: Protein Purification. Springer Science & Business Media, 2013, ISBN 978-1-4757-2333-5 , p. 81
- ↑ Data sheet ammonium sulfate (PDF) from Merck , accessed on January 19, 2011.
- ↑ PAETEC Formula Collection Edition 2003, p. 116.
- ↑ Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: November 2018. (PDF 1753 kB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, November 2018, accessed on March 27, 2019 (English, Arcanite see p. 11).
- ↑ Process for the production of high-purity ammonium sulfate and calcite using gypsum


