Ammonium hydrogen sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 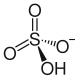
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium hydrogen sulfate | |||||||||||||||
| other names |
Ammonium bisulfate |
|||||||||||||||
| Molecular formula | NH 4 HSO 4 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 115.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.79 g cm −3 (at 20 ° C) |
|||||||||||||||
| Melting point |
120 ° C (decomposition) |
|||||||||||||||
| solubility |
easily in water (115.1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium hydrogen sulfate is an ammonium salt of sulfuric acid . It has the formula NH 4 HSO 4 .
Extraction and presentation
Ammonium bisulfate formed during decomposition of ammonium sulfate at temperatures above 100 ° C, whereby ammonia is released. Ammonium hydrogen sulfate is produced in large quantities as a separation product in activated coke filter systems for industrial exhaust gases containing sulfur dioxide . The filters are charged with oxygen, ammonia and water vapor, whereby hydrogen sulphate and ammonium sulphate are formed via sulfuric acid:
properties
Ammonium hydrogen sulfate forms white, monoclinic, hygroscopic crystals. The substance decomposes at temperatures above 120 ° C, releasing sulfur oxides, nitrogen oxides and ammonia . A solution of 100 g of ammonium hydrogen sulfate in 1 l of water has a pH value of 1 at 20 ° C , i.e. it is in the strongly acidic range.
use
It is used for the production of ammonium peroxodisulphate , as an additive in nitrogen fertilizers, for fighting forest fires as a viscous solution / suspension in water and for the production of ammonium alum and flame retardants for cellulose products.
safety instructions
Ammonium hydrogen sulfate is classified as slightly hazardous to water ( WGK 1).
Individual evidence
- ↑ a b c d e f Entry on ammonium hydrogen sulphate in the GESTIS substance database of the IFA , accessed on February 14, 2017(JavaScript required) .
- ↑ a b data sheet ammonium hydrogen sulfate (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ Heinz Brauer: Handbook of environmental protection and environmental protection technology: Volume 3: additive environmental protection: treatment of exhaust air and exhaust gases. Springer, 1996, ISBN 9783540580607 , p. 577.
- ^ Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals. 4th edition, Springer, 1997, ISBN 9783540600350 , p. 596.
- ^ Arnold Willmes: Pocket book chemical substances: elements - inorganics - organics - natural substances - polymers. 3rd edition, Harri Deutsch Verlag, 2007, ISBN 9783817117871 , p. 115.
- ↑ Patent.de: Process for the production of granulated ammonium sulfate. Patent DE4126806A1, February 11, 1993.



