Ammonium peroxodisulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
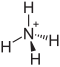  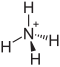
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium peroxodisulfate | |||||||||||||||
| other names |
Ammonium persulfate |
|||||||||||||||
| Molecular formula | (NH 4 ) 2 S 2 O 8 | |||||||||||||||
| Brief description |
monoclinic, colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 228.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.98 g cm −3 |
|||||||||||||||
| Melting point |
120 ° C (decomposition) |
|||||||||||||||
| solubility |
light in water (620 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium peroxodisulfate is a salt-like chemical compound made up of ammonium and peroxodisulfate ions . It has the formula (NH 4 ) 2 S 2 O 8
presentation
Ammonium peroxodisulfate is produced by anodic oxidation of ammonium sulfate or ammonium hydrogen sulfate .
properties
Ammonium peroxodisulfate is a salt of peroxodisulfuric acid . It forms white crystals with light, green fluorescence . Ammonium peroxodisulphate is a powerful bleaching and oxidizing agent as well as a strong radical generator . It must be stored in a dry place, as it decomposes in the presence of moisture or in warm, aqueous solutions, releasing ozone . Peroxodisulfates decompose when heated to form sulfate radicals . Below is the decomposition of peroxodisulfate ion into two sulfate radicals.
application
Ammonium peroxodisulfate is a polymerization initiator in the manufacture of polyacrylamide gels and is also used as a thermal radical initiator in the manufacture of superabsorbents based on acrylic derivatives. The sulfate radicals formed during the decomposition can open the same through an electrophilic attack on carbon atoms with double bonds and thus start a chain reaction. It can also be used as an oxidizing agent and for etching printed circuit boards. In chemical analysis it is used to separate manganese and chromium . In photo development it is an attenuator .
Aqueous solutions of ammonium peroxodisulfate are used for macro-etching of steel materials, for example to make segregation structures visible on continuously cast slabs .
Individual evidence
- ↑ a b c d e f Entry on ammonium peroxodisulphate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on ammonium peroxodisulfate. In: Römpp Online . Georg Thieme Verlag, accessed on April 13, 2014.
- ↑ Entry on Diammonium peroxodisulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 390-1.
- ^ Arnold Willmes: Pocket book chemical substances: elements - inorganics - organics - natural substances - polymers. 3rd edition, Harri Deutsch Verlag, 2007, ISBN 978-3-8171-1787-1 , p. 115



