Peroxodisulfates
Peroxodisulfates (outdated persulfates ) are the salts of peroxodisulfuric acid (H 2 S 2 O 8 ). Most peroxodisulfates are extremely easily soluble, including lead peroxodisulfate and barium peroxodisulfate.
Well-known salts are the readily crystallizing compounds potassium peroxodisulfate (K 2 S 2 O 8 ) and ammonium peroxodisulfate ((NH 4 ) 2 S 2 O 8 ). Peroxodisulfates arise z. B. in the anodic oxidation of sulfates . They are very strong oxidizing agents that z. B. can oxidize chromium salts to dichromate and manganese salts to permanganate.
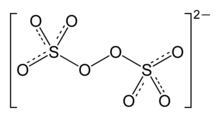
Peroxodisulfates contain the peroxodisulfate ion S 2 O 8 2− = [O 3 S – O – O – SO 3 ] 2− , which contains two SO 4 tetrahedra linked by an O – O bridge .
Peroxodisulfates decompose when heated, forming sulfate radicals :

Individual evidence
- ^ Meyer's Large Conversational Lexicon . A reference book of general knowledge. 6th edition. tape 19 . Bibliographisches Institut, Leipzig and Vienna 1909, p. 860 ( online at Zeno.org ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 81–90. Edition. Walter de Gruyter, Berlin 1976, ISBN 3-11-005962-2 , p. 346.