Hypervalence
As hypervalence [Greek hyper: over, too much, see list of Greek prefixes ] describes the positive deviation from the valence structure theory , according to which the outer shell of an atom can hold more than the eight electrons possible according to the noble gas configuration of s and p orbitals ( octet rule ). It occurs in compounds of heavy main group elements of the 3rd to 8th main group with strong electron acceptors such as oxygen or fluorine . For example in selenium halides such as selenium hexafluoride (SeF 6 ) or iodine fluorides (e.g. IF 5 , IF 7 ), generally in many noble gas and interhalogen compounds . Until recently, it was assumed that the d orbitals of the central atom were involved, but a description by an electron-rich multi-center bond is now preferred.
Examples
Hypervalent iodine compounds
In synthetic organic chemistry, hypervalent iodine compounds are widely used as mild oxidizing agents. These "modern" oxidizing agents include a:
| Iodosylbenzene | Di-acetoxy-iodobenzene (DIB) | Dess-Martin-Periodinane (DMP) | 2-iodoxybenzoic acid (IBX) |
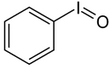 |
 |
 |
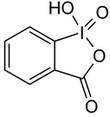
|
Dess-Martin-Periodinan is used in particular in the so-called Dess-Martin oxidation . There are also reagents with hypervalent iodine that are used for the electrophilic introduction of iodine (attack of I + on nucleophiles , e.g. C = C double bonds ):
| Di- sym- collidin-iodonium perchlorate |

|
Individual evidence
- ↑ Entry on hypervalency . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.HT07054 Version: 2.3.2.
- ↑ a b c d V. V. Zhdankin, PJ Stang: In: Chemical Reviews . 102, 2002, pp. 2523-2584.
- ↑ a b c d T. Wirth: In: Angew. Chem. 117, 2005, pp. 3722-3731.
- ↑ a b R. D. Richardson, T. Wirth: In: Angew. Chem. 118, 2006, pp. 4510-4512.
- ^ DA Griffith, SJ Danishefsky : In: J. Am. Chem. Soc. 112, 1990, pp. 5811-5819.