Oxidation by hypervalent iodine reagents
The oxidation by hypervalent iodine reagents comprising the Dess-Martin oxidation and the IBX oxidation. These are chemical reactions and describe the oxidation of primary or secondary alcohols by the oxidizing agent IBX ( 2-iodoxybenzoic acid ) or Dess-Martin-Periodinane .
IBX oxidation
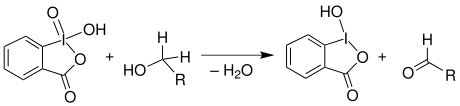
The IBX oxidation , in the organic chemistry used to oxidize primary alcohols to aldehydes. 2-Iodoxybenzoic acid, or IBX for short, is a molecule that is sensitive to heat and shock. In addition, it can not be dissolved in many solvents .
mechanism
The reaction takes place in two sub-steps. First, the alcohol to be oxidized attacks nucleophilically the iodine atom of the IBX 1 to, wherein a water molecule is split off. This is followed by the electron rearrangements shown by arrows in the figure. In this way the bond between the former primary alcohol and the iodine atom is broken, which is then reduced. The alcohol used is oxidized to the corresponding aldehyde 4 .
Dess-Martin oxidation
The Dess-Martin oxidation is a name reaction in organic chemistry and named after the American chemists Daniel Benjamin Dess and James Cullen Martin . The reaction serves to oxidize primary and secondary alcohols to aldehydes or ketones . As oxidant is Dess-Martin periodinane used. It is produced by reacting IBX in DMSO with the help of acetic anhydride to form Dess-Martin periodinane.
mechanism
The reaction is similar to that of IBX oxidation. At the beginning, the iodine atom of compound 1 is attacked by the alcohol in a nucleophilic manner, with cleavage of a molecule of acetic acid (AcOH). In an α- H - elimination of the alcohol is then oxidized to release a further molecule of acetic acid. The iodine is in this case by the oxidation state + V to + III reduced . It strives to absorb electrons and therefore functions here as an electron sink. A ketone 4 is formed .
Above all, the Dess-Martin oxidation is a very selective and mild oxidation that represents an alternative to the Swern oxidation , Jones oxidation or Pfitzner-Moffatt oxidation , but is one of the reactions that take place with low atom economy .
Reaction conditions
Typically, the reaction can be carried out at room temperature in a solvent such as chloroform or dichloromethane using only 1.1 equivalents of the periodinane reagent. The reaction time is about 0.5 to 2 hours. Working up takes place by means of basic hydrolysis. A newer variant uses the ionic liquid butylmethylimidazolium tetrafluoroborate (BMIM BF4) as a solvent .
Individual evidence
- ^ DB Dess, JC Martin: Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones in J. Org. Chem. 48 (1983) 4155-4156. doi : 10.1021 / jo00170a070 .
- ↑ PJ Stang, VV Zhdankin: Organic Polyvalent Iodine Compounds in Chem. Rev. 96 (1996) 1123-1178. doi : 10.1021 / cr940424 + .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis ; Elsevier Academic Press, Burlington-San Diego-London 2005, 1st edition; ISBN 0-12-369483-3 .
- ^ SD Meyer, SL Schreiber: Acceleration of the Dess-Martin Oxidation by Water in J. Org. Chem. 59 (1994) 7549-7552. doi : 10.1021 / jo00103a067 .
- ↑ JS Yadav, BVS Reddy, AK Basak, AV Narsaiah: Recyclable 2nd generation ionic liquids as green solvents for the oxidation of alcohols with hypervalent iodine reagents in Tetrahedron 60 (2004) 2131-2135. doi : 10.1016 / j.tet.2003.12.056 .