Jones oxidation
The Jones oxidation is a chemical reaction of organic chemistry that the oxidation of alcohols and aldehydes is used. Primary alcohols are first oxidized to aldehydes and then to carboxylic acids, secondary alcohols to ketones. Chromium (VI) oxide serves as the oxidizing agent , which is reduced to chromium (IV) and then disproportionated to chromium (III) and chromium (VI). The reaction is carried out in concentrated sulfuric acid, usually in the presence of acetone (Jones reagent). In contrast to the Swern oxidation and the Dess-Martin oxidation , the alcohol used must be stable in a strongly acidic medium.
The reaction was introduced by the British chemist Ewart Jones (1911-2002).
Overview reaction
Primary alcohols are oxidized to carboxylic acids via the stage of the mostly non-isolable aldehydes:
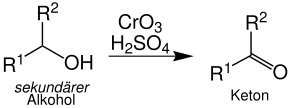
Secondary alcohols are oxidized to ketones. The organyl radicals R 1 and R 2 can be identical or different:
mechanism
The mechanism of Jones oxidation is not fully understood. The mechanism given here is only one of several possible options. The chromium trioxide is first protonated by the sulfuric acid and then reacts with the alcohol (a secondary alcohol in the example) to form an oxonium ion. The oxonium ion rearranges through a 1,3 proton shift. The alcohol is deprotonated and α-H elimination occurs, with the chromium species being split off and a ketone being formed.
use
The Jones oxidation can only be used for the synthesis of ketones and acids; stopping the reaction at the aldehyde stage is not possible. Double bonds present in the substrate are usually not attacked.
criticism
Jones oxidation is a laboratory process and is only rarely used in industry, since chromium (VI) oxide is very toxic , carcinogenic and mutagenic , and the chromium waste produced also has to be disposed of in a costly manner. In addition, the Jones oxidation is one of the reactions with low atom economy , since the quantitative ratio between the target compound (carboxylic acid or ketone) and waste materials is very unfavorable.
swell
- Kenneth Bowden, IM Heilbron, ERH Jones, BCL Weedon: Researches on acetylenic compounds. Part I. The preparation of acetylenic ketones by oxidation of acetylenic carbinols and glycols . In: Journal of the Chemical Society (Resumed) . 1946, p. 39-45 , doi : 10.1039 / JR9460000039 .
- Reinhard Brückner : reaction mechanisms. 3. Edition. Elsevier, Munich 2004, ISBN 3-8274-1579-9 , pp. 742 f.
Individual evidence
- ^ Zerong Wang: Comprehensic Organic Name Reactions and Reagents , Volume 2, Wiley, 2009, pp. 1564–1568, ISBN 978-0-471-70450-8 .