Poison
As poison ( medium high German pollutant , althochdeutsch handover ) or toxic substance is referred to a material , the living things on their metabolic processes , by penetration into the organism of a certain, low dose may cause damage. As the amount of exposure to an active ingredient increases, the likelihood that health damage will occur through poisoning increases . From a certain dose range onwards, almost every substance can be classified as poisonous ( toxic ).
The scientific discipline that deals with the study of toxic substances, their effects in different dose ranges and the treatment of poisoning is toxicology . It deals with substances, mixtures of substances, animals, plants and microorganisms and with the biochemical mechanisms of toxic effects in relation to quantitative aspects.
The damage caused by a poison can be temporary impairment, permanent damage, or death. In the case of persistent harmful effects of poison, one speaks of chronic poisoning , in the case of toxic effects that immediately lead to damage, one speaks of acute poisoning .
As hazardous substances , poisons are divided into very toxic and toxic as well as harmful to health (formerly less toxic ), depending on the amount of action .
etymology
The word poison is a Germanic abstract formation ( * gef-ti- ) with a t suffix - and the resulting change from b to f - the Indo-European root of the word give . The original meaning "gift, present, donation", which poison still had in Goethe , has now disappeared in German (while it lives on in English "gift") and has only been preserved in the dowry ("marriage property of the bride, trousseau") .
The change in meaning from “gift” to “deadly gift, poison”, first documented in Old High German by Notker , is later influenced by the Greek-late Latin word dose , which means gift, “gift, certain amount of medicine”, but also as a covering ( euphemistic) expression for "poison" is used.
But even the Greek expression pharmacon in Homer stood for both the healing effect and the harmful effect of a substance and in Galenos there are medicines ( pharmaka ) whose effect as a poison depends on the dose.
Poison retains the originally feminine gender in both meanings for the time being, then becomes a “harmful substance” first masculine (beginning of the 15th century), later neuter (mid-16th century). The latter became more and more popular in the 18th century, but Schiller wrote in Kabale und Liebe in 1784 (5th act, 7th scene): I still don't feel the poison .
Related uses: Old High German (9th century), Middle High German, Middle Low German gift (feminine) 'the giving, gift, present, gift', Middle Dutch ghifte , ghichte , Dutch gift (feminine) 'Gabe, Gift', Old English gift , gyft (feminine , neutrum) 'gift, reward, bride price', plural 'wedding', Old Norse gipt , gift (feminine) 'gift, luck, marriage (of the woman)', Gothic fragifts (feminine) 'award', plural 'engagement'.
Delimitation of the term
In general, there is a difficult distinction between pollutants and toxins .
Toxins or waste products excreted by living beings are called toxins in toxicology . Pathogenic bacteria cause damage through the effects of their toxins. The characteristic clinical pictures in bacterial infections are caused by the action of bacterial toxins.
- In gram-negative bacteria, these toxins are part of the cell membrane. They are released as endotoxins when the bacterial cells die .
- In gram-positive bacterial pathogens, the toxins arise in the intermediate metabolism. They are excreted as exotoxins .
Toxoids are detoxified (inactivated) toxins, but they can still trigger an immune response in the vaccinated body. Toxoid vaccines are used in vaccinations against diphtheria and tetanus .
Viruses are pathogens , but they are not toxic themselves. Substances or objects that only damage a living being mechanically or through radiation are also not considered poison.
Toxicity
The tolerance of a substance is different for many living beings or groups of living beings. In principle, all substances supplied to the organism above a certain dose can cause damage and are therefore to be regarded as toxic from this effective level. Paracelsus wrote in 1538: “All things are poison, and nothing is without poison; the dose alone makes sure that a thing is not poison. "
Toxicity is a measure of the harmful or lethal effect of a chemical substance or a physical effect (e.g. radiation; ionizing radiation, radiation exposure) on a living organism. A distinction is made between acute toxicity, which has an immediate effect, chronic toxicity, in which damage only occurs after a long period of exposure, and ecological toxicity (ecotoxicology), which has an impact on entire populations or ecosystems. The acute toxicity is determined by the amount (dose) of a poison required on average until a certain toxic effect is achieved, given either as the amount of poison per kg of body weight or in the form of a concentration-time product ( Haber's product ) in mg min −1 m - 3 . Chronic toxicity describes the toxic effects of a substance with long-term regular application of a certain dose. The enrichment (accumulation) of many pollutants in the air and soil falls under the field of ecotoxicology.
The absolute magnitude of the toxicity (toxic dose) depends on a number of factors. The most important are the type and location of the poison application ( inhalation , oral , subcutaneous or percutaneous , intramuscular , intravenous or intraarterial , intraperitoneal, etc.), the application vehicle (e.g. solvent), the physical condition of a living being (type, age and gender , individual characteristics of the living being, predisposition , state of health and living conditions) and chronobiological factors (time of application). In order to be able to compare toxicity information, these parameters must therefore always be given.
Nanotoxicology deals with the effects of nanoparticles, including nanomaterials, on living organisms. Due to miniaturization, nano-objects sometimes show significantly different physical and chemical properties compared to their original material. The smaller a particle, the greater its toxicity. Nanoparticles can cause bronchial and pulmonary inflammatory reactions , and pulmonary fibrosis has also been described in isolated cases .
The effects of toxic substances can be partially neutralized or mitigated by natural or artificially produced antidotes .
Poisonous effect
Toxic effect on humans
Poisons attack different receptors in the organism. Often affected organs in acute poisoning are the liver ( hepatotoxins , e.g. from paracetamol ), kidneys ( nephrotoxins ) as well as the brain and nerves ( neurotoxins such as botulinum toxin and warfare agents such as VX , sarin or soman ). Some toxins affect internal respiration , such as nitrites and carbon monoxide , which block hemoglobin , or potassium cyanide (cyanide), which blocks the cells' respiratory chain .
In order to be able to compare the toxicity of toxins with one another, animal experiments are used under standardized conditions. The frequently given LD 50, for example, indicates the amount of substance, based on body weight, which leads to death in half of a test animal population. LD stands for lethal dose .
Some of the most well-known toxins are grouped under the collective term botulinum toxin , and they can be found in spoiled canned meat and fish or in cheese.
Concentration poisons and summation poisons
According to the behavior of the toxin at the receptors, two types of toxins are distinguished:
- In the case of a concentration poison, the effect increases as the concentration of the poison at the receptors increases. If the toxin, for example through metabolic processes or exhalation, is completely broken down again - without having damaged the blocked receptors - the effect also completely subsides.
- In the case of a summation poison, also known as an accumulation poison or ct poison, the poison causes an irreversible change in the receptors. The effects persist even after the active substance is excreted from the body. With a further dose, the toxin molecules can irreversibly damage some of the remaining receptors. The individual effects can add up in this way. The absorption of toxins can take place continuously or in batches. The effect (W) results from the product of concentration (c) and exposure duration (t) as W = c · t (see Habersche rule ).
- Examples
- Tobacco smoke contains nicotine , which is very toxic in high concentrations . Nicotine is a typical concentration poison . It reversibly blocks the nicotinic acetylcholine receptors . After a short time, the receptors are released again. Even when taken over many years, nicotine shows only minor chronic damage to the organism in low doses.
- Tobacco smoke also contains several carcinogenic compounds. The best known is benzo [ a ] pyrene , which has a lower acute toxicity than nicotine, but is a typical summation poison . The oxidation product benzo [ a ] pyrene-7,8-dihydroxy-9,10-epoxide, formed in the organism from benzopyrene, causes, when taken in very small doses over many years, a considerably increased risk of lung cancer and other types of cancer, since it is associated with a Part of the genetic material DNA reacts.
Examples of different toxic effects
- Poison "cocktails", as they are sometimes put together with the intention of murder or suicide , are usually more "poisonous" than the sum of the individual substances ("potentiation"). This also applies to the combination of sub-toxic quantities of environmentally hazardous substances , which together can have a harmful effect.
- Metallic mercury is less toxic if swallowed than if the vapors are inhaled .
- A dose of ethanol that is consumed and tolerated in the form of beer in the course of an evening (i.e. subacute) can lead to more pronounced and potentially dangerous symptoms of poisoning if consumed as schnapps .
- Ingesting 10 liters of water at once (distilled or not) can be fatal to an adult. Hyponatremia occurs (insufficient supply of sodium due to osmotic withdrawal). However, this is not a toxic effect of the water itself, but a harmful dilution effect.
- An organism that has been damaged by illness reacts more sensitively to poisons than that of a healthy person.
- A dose of digitoxin that is therapeutic in an adult can be fatal to a child or the elderly.
- Ethanol is fatal to people with reduced or altered alcohol dehydrogenase in a much lower dose.
- The LD 50 value for DDT is 113 mg / kg in rats , but only 1 mg / kg body weight in flies .
- 2,3,7,8-Tetrachlorodibenzodioxin is acutely fatal in sheep; in humans, the same concentration only leads to the development of chloracne .
- The theobromine of chocolate (or cocoa) is for dogs and cats toxic, see theobromine poisoning .
- Repeated poisoning leads to the development of tolerance for many substances . For example, there used to be arsenic eaters who in some cases ingested several times the usually acutely lethal dose of arsenic (As 2 O 3 ) without (acute) impairment. Thallium salts have a similar effect on the human organism as arsenic . A closer example is heroin (an opioid ), to which humans develop a pronounced tolerance.
- White germer , a highly toxic plant for most mammals, is consumed by red deer during the rut.
- Poisoning with sleeping pills sometimes leads to death through disturbances in temperature regulation with cooling of the organism. If the cooling is counteracted (blanket, heating), an overdose can be tolerated that would have been fatal in the open air.
Classification of poisons
Toxic substance as a hazardous substance
While generally toxic pollutants as dangerous for the environment are classified (N) pulps are sent to the effect on people as hazardous substances in very toxic (T +), toxic (T) and harmful classified (Xn) (obsolete "less toxic").
| Hazard symbol with hazard designation |
Code letter |
Classification for hazard symbols | Examples | |
|---|---|---|---|---|
| very poisonus | T + | if in very small quantities they lead to death or acute or chronic damage to health if they are inhaled, swallowed or absorbed through the skin . The following limit values apply | Atropine , sarin , thallium | |
| toxic | T | if they can cause death or acute or chronic damage to health in small quantities if inhaled, swallowed or absorbed through the skin; All CMR substances are also marked with a T. The following limit values apply
|
Methanol , carbon tetrachloride | |
| harmful to health | Xn | if they can cause acute or chronic damage to health if inhaled, swallowed or absorbed through the skin. The following limit values apply
|
Dichloromethane , potassium chlorate | |
According to the more recent classification according to the globally harmonized system for classification and labeling of chemicals, there is a division into acutely toxic (symbol 06), health hazard (symbol 08) and various other health hazards (symbol 07).
| GHS symbol with signal word |
Classification for hazard symbols | Examples | |
|---|---|---|---|
| danger | if they can cause death or acute or chronic damage to health in small quantities if inhaled, swallowed or absorbed through the skin . The following limit values apply
|
Atropine , sarin , thallium , methanol , carbon tetrachloride | |
| danger | if they can cause acute or chronic damage to health if inhaled, swallowed or absorbed through the skin. The following limit values apply
or with carcinogenic or allergenic substances |
Dichloromethane | |
| The “thick exclamation mark symbol” is used for the sole or additional identification of various categories that were previously mainly covered by the hazard symbol Xi for irritant. It may also be omitted. The signal word is selected depending on the context. | Potassium chlorate | ||
The regulations are EU-wide compliant. According to the Swiss Poison Act, the classification into poison classes was made , but the EU hazard symbols have also been in effect since 2005 .
As dangerous goods in transport, which are regulated by the ADR on the road , toxic substances have the dangerous goods class 6.1 - Toxic substances or, in the case of gases, 2 with the degree of danger T (toxic); TF (toxic and flammable); TC (toxic and corrosive); TO (toxic and oxidizing); TFC (toxic, flammable and oxidizing); TOC (toxic, oxidizing and corrosive) and a number to identify the hazard (Kemler number) 6.
| Class of dangerous goods | classification | Examples | ||
|---|---|---|---|---|
 |
Class 6.1 | Toxic substances | Substances which are known from experience or which can be assumed based on animal experiments that, if inhaled, swallowed or come into contact with the skin, a single or brief exposure in relatively small quantities can lead to health problems or human death. | Hydrogen cyanide ( hydrogen cyanide ), arsenic , pesticides |
 |
Class 2, hazard groups T, TF, TC, TO, TFC, TOC | Gases (toxic) | Gases, a) who are known to be so toxic and corrosive to humans as to pose a health hazard; or |
Chlorine gas , hydrogen chloride , sulfur dioxide |
As a toxic substance wear toxins typically the R-sets 20-28 ( Harmful / Toxic / Very toxic by inhalation / in contact with the skin / if swallowed ), R29, 31, 32 ( toxic gas on contact with other substances), as well as R50–59 (dangerous for the environment ). But a number of other R-phrases also describe toxic effects in a medical or legal sense (irritant effect, cancer risk, genetic damage, ...).
A list of the toxic and very toxic substances described in Wikipedia can be found in the category: Toxic substance .
Legal definition
According to the prevailing view, a poison is any organic or inorganic substance which, according to its type, the amount introduced, the form in which it was introduced and the nature of the victim's body, is likely to damage the health through chemical or chemical-physical effects.
- Taught is a poison when a body fabric relationship was prepared.
The legislature expressly refers to the classification as a hazardous substance (e.g. Section 3, Paragraph 1, Item 6 and 7 of the ChemG 1996 , Austria), whereby substances designated as harmful are also included in particular (e.g., Section 35, Item 1 of the ChemG 1996). According to both the chemical laws and the hazardous substance ordinances, a sufficiently well-founded suspicion of toxicity is enough to classify a substance as a poison .
Bringing poison is punished (in Germany according to Section 224, Paragraph 1, No. 1 Alt. 1 StGB ) as dangerous bodily harm .
LD 50 table and logarithmic poison scale
Table of the LD 50 values of some substances in different types:
| substance | Species, route of administration | LD 50 {LC 50 } |
LD 50 : g / kg {LC 50 : g / L} standardized |
Individual proof |
|---|---|---|---|---|
| water | Rat, oral | > 90 g / kg | > 90 | |
| Sucrose (sugar) | Rat, oral | 29.7 g / kg | 29.7 | |
| Monosodium glutamate (MSG) | Rat, oral | 16.6 g / kg | 16.6 | |
| Vitamin C (ascorbic acid) | Rat, oral | 11.9 g / kg | 11.9 | |
| urea | Rat, oral | 8,471 mg / kg | 8,471 | |
| Cyanuric acid | Rat, oral | 7,700 mg / kg | 7.7 | |
| Cadmium sulfide | Rat, oral | 7,080 mg / kg | 7.08 | |
| Ethanol | Rat, oral | 7,060 mg / kg | 7.06 | |
| Isopropylmethylphosphonic acid (IMPA, metabolite of sarin ) | Rat, oral | 6,860 mg / kg | 6.86 | |
| melamine | Rat, oral | 6,000 mg / kg | 6th | |
| Melamine cyanurate | Rat, oral | 4,100 mg / kg | 4.1 | |
| Sodium molybdate | Rat, oral | 4,000 mg / kg | 4th | |
| Sodium chloride (table salt) | Rat, oral | 3,000 mg / kg | 3 | |
| Paracetamol (acetaminophen) | Rat, oral | 1,944 mg / kg | 1,944 | |
| Delta-9-tetrahydrocannabinol (THC) | Rat, oral | 1,270 mg / kg | 1.27 | |
| arsenic | Rat, oral | 763 mg / kg | 0.763 | |
| Alkyldimethylbenzalkonium chloride (ADBAC) | Rat, oral fish, immersion aquatic invertebrates, imm. |
304.5 mg / kg {0.28 mg / L} {0.059 mg / L} |
0.3045 {0.00028} {0.000059} |
|
| Coumarin (from Cinnamomum aromaticum and other plants) | Rat, oral | 293 mg / kg | 0.293 | |
| Acetylsalicylic acid (ASA) | Rat, oral | 200 mg / kg | 0.2 | |
| caffeine | Rat, oral | 192 mg / kg | 0.192 | |
| Arsenic sulfide | Rat, oral | 185-6,400 mg / kg | 0.185-6.4 | |
| Sodium nitrite | Rat, oral | 180 mg / kg | 0.18 | |
| Uranyl acetate dihydrate | Mouse, oral | 136 mg / kg | 0.136 | |
| Bisoprolol | Mouse, oral | 100 mg / kg | 0.1 | |
| Mustard gas | Man, dermal | 100 mg / kg | 0.1 | |
| Cobalt chloride | Rat, oral | 80 mg / kg | 0.08 | |
| Cadmium oxide | Rat, oral | 72 mg / kg | 0.072 | |
| Sodium fluoride | Rat, oral | 52 mg / kg | 0.052 | |
| Pentaborane | Human, oral | <50 mg / kg | <0.05 | |
| Capsaicin | Mouse, oral | 47.2 mg / kg | 0.0472 | |
| Mercury (II) chloride | Rat, dermal | 41 mg / kg | 0.041 | |
| Sarin | Human, dermal mouse, subcutaneous |
28 mg / kg 172.23 µg / kg |
0.028 0.00017 |
|
| Lysergic acid diethylamide (LSD) | Rat, intravenous | 16.5 mg / kg | 0.0165 | |
| Arsenic trioxide | Rat, oral | 14 mg / kg | 0.014 | |
| arsenic | Rat, intraperitoneally | 13 mg / kg | 0.013 | |
| Nicotine | Human, oral | 6.5-13.0 mg / kg | 0.0065-0.013 | |
| Sodium cyanide | Rat, oral | 6.4 mg / kg | 0.0064 | |
| White phosphorus | Rat, oral | 3.03 mg / kg | 0.00303 | |
| Strychnine | Human, oral | 1-2 mg / kg | 0.001 | |
| Cantharidin | Human, oral | 0.5 mg / kg | 0.0005 | |
| Aflatoxin B1 (from Aspergillus flavus ) | Rat, oral | 0.48 mg / kg | 0.00048 | |
| Venom of the Brazilian wandering spider | Rat, subcutaneous | 134 µg / kg | 0.000134 | |
| Inland Taipan Venom ( Venomous Australian Snake) | Rat, subcutaneous | 25 µg / kg | 0.000025 | |
| Ricin | Rat, intraperitoneal rat, oral |
22 µg / kg 20-30 mg / kg |
0.000022 0.02 |
|
| 2,3,7,8-tetrachlorodibenzodioxin (TCDD, a dioxin ) | Rat, oral | 20 µg / kg | 0.00002 | |
| VX | Human, oral, inhalation, skin / eye absorption | 2.3 μg / kg (estimated) | 0.0000023 | |
| Batrachotoxin (from poison dart frogs ) | Human, subcutaneous | 2-7 µg / kg | 0.000002 | |
| Abrin | Mouse, intravenous human, inhalation human, oral |
0.7 µg / kg 3.3 µg / kg 10-1000 µg / kg |
0.0000007 0.0000033 0.00001-0.001 |
|
| Maitotoxin | Mouse, intraperitoneally | 0.13 µg / kg | 0.00000013 | |
| Polonium-210 | Human, inhalation | 10 ng / kg (estimated) | 0.00000001 | |
| Botulinum toxin (botox) | Human, oral, injection, inhalation | 1 ng / kg | 0.000000001 |
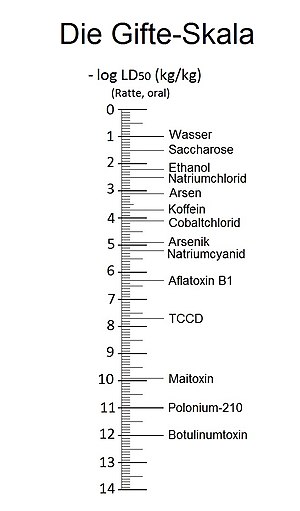
The LD 50 values have a very wide range. The botulinum toxin as giftigster known material has a LD 50 ng value of 1 / kg, while the fabric ungiftigste water a LD 50 value of greater than 90 g / kg did. That's a difference of about 1 in 100 billion, or 11 orders of magnitude. As with all measured values that differ by many orders of magnitude, a logarithmic view is recommended. Well-known examples are the indication of the earthquake strength using the Richter scale , the pH value , as a measure of the acidic or basic character of an aqueous solution or the volume in dB . In this case, the negative decadic logarithm of the LD 50 values, which is given in standardized form in kg per kg of body weight, is used.
- -log LD 50 (kg / kg) = value
The dimensionless value found can be entered on a toxin scale. Water as the most important substance has the catchy value 1 in the toxicity scale obtained in this way.
Poisons effective in humans (examples)
| substance | Exposure path | Amount in mg kg −1 | source |
|---|---|---|---|
| Ethanol | orally | 1400 | |
| phosphorus | orally | 1.4 | |
| bromine | orally | 14th | |
| nitric acid | orally | 430 | |
| phenol | orally | 140-1400 | |
| Pyridine | orally | 500 | |
| Atropine | unknown | 0.143 | |
| Potassium cyanide | orally | 2.857 | |
| quinine | unknown | 294 | |
| Mustard gas | percutaneous | 64 | |
| Ciprofloxacin | orally | 5.714 | |
| Sodium cyanide | orally | 2.8 | |
| Parathion | orally | 0.17 | |
| Phencyclidine | orally | 14th | |
| Mercury (II) chloride | orally | 1 | |
| Diethylene glycol | orally | about 1000 | |
| Cantharidin | orally | 0.03-0.5 | |
| Dichlorvos | orally | 50 | |
| Lewisite | percutaneous | 37.6 | |
| Picrotoxin | orally | 0.357 | |
| Lead (II) carbonate | orally | 571 | |
| Heptabarbital | orally | 50 | |
| Gyromitrin | orally | 20th |
Toxins
Toxins are poisons that are synthesized by living things.
Poisons produced by the human organism:
- Aconitine (monkshood)
- Colchicine (autumn crocus)
- Coniin (hemlock)
- Curare
- Digitoxin (thimble)
- Nicotine (tobacco plant)
- Ricin (castor oil)
- Strychnine (nugget)
- Taxanes (yew trees)
- Tropane alkaloids (deadly nightshade, thorn apple, angel's trumpet, henbane)
Poisons produced by microorganisms :
-
Bacterial toxins
- Botulinum toxin ( Clostridium botulinum )
- Exotoxin A ( Pseudomonas aeruginosa )
- Shiga toxin ( Shigella dysenteriae )
- Vero toxin ( Escherichia coli )
- Brevetoxin ( Karenia brevis , a seaweed)
- Mycotoxins (mold toxins)
Mushroom poisons (poisonous large mushrooms ):
- Amatoxins (death cap mushroom)
- Amphibian poisons
- Bee venom
- Fish poison (puffer fish)
- Hornet poison
- animal poison darts (poison dart frog, etc.)
- Snake venom
- Poisons of the scorpions
- Spider venom
- Male platypus poison
- Poisonous marine invertebrates (such as the wasps Chironex fleckeri and Chiropsalmus quadrigatus and the blue-ringed octopus )
Other poisons
- ammonia
- Arsenic and arsenic
- beryllium
- Carbon monoxide
- Hydrogen sulfide
- most heavy metals , e.g. B. cadmium , mercury or plutonium
- Phosphine
- Hydrogen cyanide ( hydrogen cyanide )
- Potassium cyanide (cyanide)
Organic compounds:
See also
- Chemicals Act (Switzerland) (also called Poisons Act )
- Poison Control
- Lethal injection , execution by poison
- Food poisoning
- List of intoxications and antidotes
literature
- Mechthild Amberger-Lahrmann, Dietrich Schmähl (Ed.): Poisons. History of toxicology. Berlin et al. 1988.
- Karsten Strey: The world of poisons. Lehmanns Media, Berlin 2015. ISBN 978-3-86541-728-2 .
- Eberhard Teuscher, Ulrike Lindequist: Biogenic poisons. Biology - Chemistry - Pharmacology. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2010, ISBN 978-3-8047-2438-9 .
- Louis Lewin: The poisons in world history , reprograph. Reprint of the edition Berlin, Springer 1920, Tosa 2007, Vienna ISBN 978-3-85003-152-3 .
- Ludwig Sacha Weilemann, Hans-Jürgen Reinecke: Emergency manual poisonings. Stuttgart, Thieme 1996. ISBN 978-3-13-102591-3 .
Web links
- Giftinfo.de, advice center for poisoning
- Swiss Toxicological Information Center
- ATSDR - ToxFAQs ™: Hazardous Substance Fact Sheets , United States Department of Health
- SuperToxic , database, Charité Berlin
Individual evidence
- ↑ Thomas Richter: Poisons. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 494 f .; here: p. 494.
- ↑ Pfeifer, Dr. Wolfgang , Etymological Dictionary of German , Deutscher Taschenbuch Verlag (dtv) Munich, 5th edition 2000, p. 449.
- ↑ The ICD-10 differentiates between poisoning (T36-T50) and toxins (T51-T65) on the one hand and everything else including radiation sickness (T66) on the other. See Chapter XIX of the ICD-10 database ( Memento from April 12, 2015 in the Internet Archive ).
- ↑ Paracelsus: The third defension because of the writing of the new recipes . In: Septem Defensiones 1538. Werke Vol. 2, Darmstadt 1965, p. 510. zeno.org .
- ↑ toxicity. In: Spectrum Academic Publishing House, Heidelberg. September 4, 2018, accessed September 2, 2019 .
- ↑ a b Toxicity. In: Spectrum Academic Publishing House, Heidelberg. December 4, 2014, accessed September 2, 2019 .
- ↑ Oberdörster G, et al .: Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. . In: Environ Health Perspect. 2005 Jul; 113 (7): 823-39. . July 11, 2005. doi : 10.1289 / ehp.7339 .
- ↑ Nanotoxicology. In: M. Müller, M. Fritz, A. Buchter, Saarland University, Central Gazette for Occupational Medicine 58 (2008) 238–252. 2008, accessed September 2, 2019 .
- ↑ University of Potsdam: Toxikodynamik ( Memento from November 17, 2012 in the Internet Archive ) (PDF; 5 kB) accessed on November 1, 2008.
- ^ Entry on nicotine in the GESTIS substance database of the IFA , accessed on March 12, 2013(JavaScript required) .
- ↑ Entry for CAS no. 50-32-8 in the GESTIS substance database of the IFA , accessed on January 15, 2008(JavaScript required) .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Verlag Helvetica Chimica Acta, Zurich 2006, page 218, ISBN 978-3-906390-29-1 .
- ↑ a b c EC Directive 67/548 / EEC Annex 6 ( Memento from March 16, 2013 in the Internet Archive ) (PDF, German) .
- ↑ a b CLP (PDF) .
- ↑ ADR 2007, Annex A, Part 2 (PDF; 323 kB) Section 2.2.2.1.3 (English).
- ↑ ADR 2007 , chapter 2.2 class specific provisions .
- ↑ ADR 2007 Annex A, Part 3, Table A: Dangerous goods list (PDF, English; 731 kB) .
- ↑ Material Safety Data Sheet Water MSDS. Section 11: Toxicological Information for the LD 50 verification. Archived from the original on September 2, 2012 ; accessed on September 5, 2019 (English).
- ↑ Safety (MSDS) data for sucrose
- ↑ Walker R, Lupien JR: The safety evaluation of monosodium glutamate . In: Journal of Nutrition . 130, No. 4S Suppl, April 2000, pp. 1049S-52S. PMID 10736380 .
- ↑ Safety (MSDS) data for ascorbic acid. Oxford University , October 9, 2005, archived from the original on February 9, 2007 ; accessed on February 21, 2007 .
- ↑ Safety (MSDS) data for urea. Section 11: Toxicological Information for the LD 50 verification. March 6, 2015, archived from the original on March 1, 2015 ; accessed on September 5, 2019 (English).
- ↑ a b c A.A. Babayan, AVAleksandryan, "Toxicological characteristics of melamine cyanurate, melamine and cyanuric acid", Zhurnal Eksperimental'noi i Klinicheskoi Meditsiny, Vol.25, 345-9 (1985). Original article in Russian.
- ↑ Data sheet cadmium sulfide at AlfaAesar, accessed on July 17, 2013 ( PDF )(JavaScript required) .
- ↑ Safety (MSDS) data for ethyl alcohol
- ↑ Francis J. Mecler: Mammalian Toxological Evaluation of DIMP and DCBP (Phase 3 - IMPA) , Final report. Edition, Litton Bionetics, Inc., May 1981: "The oral LD50 values for the test material, IMPA, were 7650 and 6070 mg / kg for male and female rats, respectively."
- ↑ Safety (MSDS) data for sodium molybdate
- ↑ Safety (MSDS) data for sodium chloride ( Memento from October 30, 2007 in the Internet Archive )
- ↑ Safety (MSDS) data for 4-acetamidophenol
- ↑ LD50 values of THC in fischer rats
- ↑ Safety data sheet arsenic .
- ↑ Frank T. Sanders: Reregistration Eligibility Decision for alkyl dimethyl benzyl ammonium chlorides (ADBAC) , United States Environmental Protection Agency
- ^ Coumarin Material Safety Data Sheet (MSDS). Retrieved September 5, 2019 .
- ↑ Safety (MSDS) data for acetylsalicylic acid
- ↑ Safety (MSDS) data for caffeine ( Memento from October 16, 2007 in the Internet Archive )
- ↑ Material Safety Data Sheet - Spent Metal Catalyst . Archived from the original on September 28, 2011.
- ↑ Safety (MSDS) data for sodium nitrite
- ^ Chemical toxicity of uranium
- ↑ Entry on bisoprolol in the DrugBank of the University of Alberta , accessed on November 18, 2019.
- ↑ Entry on poison in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Safety (MSDS) data for cobalt (II) chloride
- ↑ Safety (MSDS) data for cadmium oxide
- ↑ Sodium Fluoride MSDS
- ^ Pentaborane chemical and safety data
- ↑ Capsaicin Material Safety Data Sheet. (PDF) sciencelab.com, 2007, archived from the original on September 29, 2007 ; accessed on July 13, 2007 .
- ↑ Entry on mercury (II) chloride in the GESTIS substance database of the IFA , accessed on December 19, 2017(JavaScript required) .
- ↑ Entry on poison in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ Science Journal. 3, No. 4, 1967, p. 33.
- ↑ Histochemical Demonstration of Calcium Accumulation in Muscle Fibers after Experimental Organophosphate Poisoning ( Memento from September 27, 2016 in the Internet Archive ). Het.sagepub.com (1990-07-01). Retrieved July 17, 2013.
- ↑ Erowid LSD (Acid) Vault: Fatalities / Deaths . Erowid.org. Retrieved July 17, 2013.
- ↑ Safety (MSDS) data for arsenic trioxide
- ↑ Safety (MSDS) data for metallic arsenic
- ↑ Mayer B: How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century . In: Archives of Toxicology . 88, No. 1, January 2014, pp. 5-7. doi : 10.1007 / s00204-013-1127-0 . PMID 24091634 . PMC 3880486 (free full text).
- ↑ Safety (MSDS) data for sodium cyanide
- ^ Hexachloroethanes. Retrieved January 3, 2014 .
- ↑ INCHEM: Chemical Safety Information from Intergovernmental Organizations: Strychnine .
- ↑ Safety (MSDS) data for aflatoxin B1 ( Memento from April 23, 2012 in the Internet Archive )
- ↑ Venomous Animals and their Venoms , vol. III, ed. Wolfgang Bücherl and Eleanor Buckley
- ↑ LD50 for various snakes . Seanthomas.net. Retrieved July 17, 2013.
- ^ Ricin (from Ricinus communis) as undesirable substances in animal feed - Scientific Opinion of the Panel on Contaminants in the Food Chain . In: EFSA Journal . tape 6 , no. 9 , 2008, p. 726 , doi : 10.2903 / j.efsa.2008.726 .
- ↑ US National Toxicology Program acute toxicity studies for Dioxin (2,3,7,8-TCDD) ( Memento from September 12, 2014 in the Internet Archive )
- ^ Toxicity of the Organophosphate Chemical Warfare Agents GA, GB, and VX: Implications for Public Protection ( Memento of December 4, 2008 in the Internet Archive )
- ↑ Brief Review of Natural nonprotein neurotoxin
- ↑ Akihiro Yokoyama, Michio Murata, Yasukatsu Oshima, Takashi Iwashita, Takeshi Yasumoto: Some Chemical Properties of Maitotoxin, a Putative Calcium Channel Agonist Isolated from a MarineDinoflagellate . In: J. Biochem. . 104, No. 2, 1988, pp. 184-187. PMID 3182760 .
- ↑ Topic 2 Toxic Chemicals and Toxic Effects ( Memento from September 29, 2007 in the Internet Archive )
- ^ Diane O. Fleming, Debra Long Hunt: Biological Safety: principles and practices . ASM Press, Washington, DC 2000, ISBN 1-55581-180-9 , p. 267.
- ↑ K. Strey: The poison scale . In: Chemistry in Our Time . 53, No. 6, December 2019, pp. 386-399. doi : 10.1002 / ciuz.201900828 .
- ↑ Raw Material Data Handbook. Vol. 1: Organic Solvents, 1974, p. 44.
- ^ Pesticide Chemicals Official Compendium , Association of the American Pesticide Control Officials, Inc., 1966 , p. 901.
- ^ WB Deichmann: Toxicology of Drugs and Chemicals , Academic Press, Inc., New York, 1969, p. 645.
- ↑ Data sheet 100% nitric acid (PDF) from Merck , accessed on January 19, 2011.
- ↑ R. Lefaux, OH Cleveland: Practical Toxicology of Plastics. Chemical Rubber Co., 1968, p. 329.
- ^ WB Deichmann: Toxicology of Drugs and Chemicals. New York 1969, Academic Press, p. 463.
- ↑ S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita, H. Ichimura: Pyridine and Pyridine Derivatives , in: Ullmann's Encyclopedia of Industrial Chemistry , 2005 , Wiley-VCH Weinheim.
- ^ Daniel Bovet , Filomena Bovet-Nitti: Structure et Activité Pharmacodynamique des Médicaments du Système Nerveux Végétatif. S. Karger, Basel 1948, p. 482.
- ↑ Entry on Potassium cyanide in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.
- ↑ Entry on quinine in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.
- ^ JM Arena, IL Springfield, CC Thomas: Poisoning; Toxicology, Symptoms, Treatments, 2nd Edition, 1970, p. 73.
- ^ Entry on Sulfur mustard in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.
- ↑ Shmuel Fuchs, Zvi Simon, Mayer Brezis: Fatal hepatic failure associated with ciprofloxacin. In: The Lancet. 343, 1994, p. 738, doi: 10.1016 / S0140-6736 (94) 91624-1 .
- ↑ Sodium cyanide data sheet (PDF) from Merck , accessed on January 19, 2011.
- ↑ Parathion data sheet at Sigma-Aldrich , accessed on May 5, 2008 ( PDF ).
- ↑ Entry on phencyclidine hydrochloride in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.
- ↑ Data sheet mercury (II) chloride (PDF) from Merck , accessed on April 24, 2010.
- ↑ BfR : Diethylene glycol (DEG) in toothpaste. ( Memento of August 27, 2010 in the Internet Archive ) (PDF; 45 kB) BfR Opinion No. 025/2008 of July 16, 2007.
- ↑ Cantharidin data sheet (PDF) from Carl Roth , accessed on December 12, 2007.
- ↑ Entry on Dichlorvos in the GESTIS substance database of the IFA , accessed on December 9, 2007 (JavaScript required)
- ^ Entry on Lewisite in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.
- ^ Deichmann, WB: Toxicology of Drugs and Chemicals . Academic Press, New York 1969, p. 476.
- ↑ Data sheet lead (II) carbonate (PDF) from Merck , accessed on January 19, 2011.
- ↑ Irving S. Rossoff: Encyclopedia of clinical toxicology: a comprehensive guide and reference. Informa Health Care, 2002, ISBN 1-84214-101-5 , p. 515.
- ↑ Entry on gyromitrin in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on May 26, 2016.