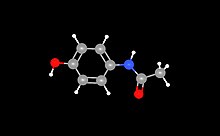
Paracetamol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Paracetamol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 9 NO 2 | ||||||||||||||||||
| Brief description |
white, odorless, monoclinic prisms |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Inhibition of cyclooxygenase-2 (COX-2) in the spinal cord |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 151.16 g · mol -1 | ||||||||||||||||||
| density |
1.29 g cm −3 (21 ° C) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
9.38 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
10 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Paracetamol is an analgesic and antipyretic drug from the group of non-opioid analgesics . In North America and Iran, the common name for the substance is acetaminophen .
The name paracetamol is derived from the chemical name par a - ( acet yl am ino) phen ol (or para - ( acet yl amino ) phen ol). The substance is a derivative of both acetic acid and the aminophenol p-hydroxyaniline. Paracetamol is used in self-medication as a single preparation or as part of various combination preparations for the symptomatic treatment of cold symptoms and pain . Since their introduction in the 1950s, almost 70 years after Harmon Northrop Morse first manufactured this active ingredient , medicines containing paracetamol - alongside those containing acetylsalicylic acid and ibuprofen - have been among the most widely used pain relievers worldwide. Paracetamol has been on the WHO list of essential drugs since 1977 .
history
The production of paracetamol as a product of the reduction of p - nitrophenol with zinc in glacial acetic acid (concentrated acetic acid) was first described in 1878 by Harmon Northrop Morse . Josef von Mering used paracetamol for the first time in medicine in 1887, but its use caused little stir. In the following years, two substances closely related to paracetamol, acetanilide and phenacetin , gained significantly more therapeutic importance .
Paracetamol was first detected in the urine of a person who had taken phenacetin in 1893. In 1899, paracetamol was also recognized as a metabolite ( metabolite ) of acetanilide - these discoveries, however, remained without resonance, so that paracetamol was still not used in medicine.

Paracetamol only became better known after the Second World War , when it was identified a second time as a metabolite of phenacetin by Bernard B. Brodie and Julius Axelrod at the New York City Department of Health in 1948 . On behalf of the government, they researched new painkillers and showed in their work that the pain-relieving effect of acetanilide and phenacetine is entirely due to the breakdown product of these substances, paracetamol. They suggested using this substance in its pure form in order to avoid the toxic side effects of the original substances .
Paracetamol was first used in a finished drug in 1955 in the USA in Tylenol Children's Elixir from McNeil Laboratories. Paracetamol has been available in tablet form with 500 mg of active ingredient since 1956 and was sold in Great Britain under the brand name Panadol , manufactured by Frederick Stearns & Co, an offshoot of Sterling Drug Inc. It was only available on prescription and was advertised as a pain reliever and fever lowering agent, which was also easy on the stomach. Acetylsalicylic acid, which was already known at the time, is less stomach-friendly. In 1958, a children's version of the preparation called Panadol Elixir came onto the market. In 1959, paracetamol was launched on the German market by the Munich company bene-Arzneimittel as the first monopreparation under the brand name ben-u-ron .
Paracetamol has been monographed in the British Pharmacopoeia , the " British Pharmacopoeia ", since 1963 . Shortly afterwards it was also included in the pharmacopoeias of other European countries.
In 1982 there was a case of product sabotage in the USA , the consequences of which prompted the responsible state authority, Food and Drug Administration , to issue stricter protective regulations. Seven patients died from poisoned paracetamol preparations. Preparations with a market value of 100 million US dollars were recalled.
After hamster purchases occurred during the COVID-19 pandemic in Germany , the amount sold in Germany was limited to one pack per patient in April 2020.
application
Application areas (indications)
Paracetamol is approved as a finished drug for the treatment of mild to moderate pain and fever. Using it is used mainly for mild headache , slight toothache , menstrual pain , sunburn and arthritis-related joint pain as well as migraine .
Paracetamol in a fixed combination with caffeine (400 mg paracetamol, 50 mg caffeine) is also approved for the treatment of mild to moderate pain . This combination is said to be 1.3 to 1.7 times more potent than paracetamol alone and enables the paracetamol dose to be reduced. Caffeine also shortens the time it takes for paracetamol to take effect. The triple combination of paracetamol with acetylsalicylic acid and caffeine also leads to increased effectiveness and is therefore recommended by the German Migraine and Headache Society as the first choice for the treatment of migraines and tension headaches .
In a fixed combination with codeine (co-codamol) or tramadol , paracetamol is approved for the treatment of moderately severe to severe pain.
Since colds can be accompanied by fever, pain in the limbs and headaches, paracetamol is approved in combination with other drugs such as antihistamines , cough suppressants , cough suppressants or vitamin C as an analgesic and antipyretic component of many so-called "flu remedies" or "cold remedies".
Contraindications (contraindications) and warnings
Paracetamol must not be used if there is a known hypersensitivity to paracetamol or if there is severe impairment of liver function due to liver failure with a Child-Pugh score of 9 or higher. In patients with liver failure with a Child-Pugh score of <9, Gilbert-Meulengracht syndrome , chronic kidney failure with a creatinine clearance of less than 10 ml / min or chronic alcohol abuse , paracetamol may only be used with special care under medical supervision and if necessary an adjusted dosage.
Long-term, high-dose, improper use of paracetamol can result in drug-induced headache . Headache and accompanying symptoms worsen after abrupt discontinuation.
pregnancy and breast feeding period
Mostly older reproduction studies and epidemiological data gave no indication of a harmful effect of paracetamol on the health of the fetus or the newborn. Numerous studies suggest a possible connection between the use of paracetamol during pregnancy and an increased incidence of asthma in children. The influence of paracetamol on haematopoietic stem cell development in the umbilical cord blood is discussed as a possible pathomechanism, which can lead to a differentiation of immune cells. For long-term use during pregnancy, there are insufficient data to assess the safety. However, a current study by the University of Oslo clearly suggests that taking paracetamol during pregnancy and breastfeeding, especially over a longer period of time, can lead to a later developmental delay in the unborn child. Over a period of nine years, over 48,000 children, including almost 3,000 pairs of siblings, were examined.
The suspicion that children who were exposed to the active ingredient paracetamol in the womb can later become behaviorally abnormal was confirmed by a study by researchers at the University of Bristol with a total of 14,500 mothers and their children. In seven-year-old children whose mothers had taken paracetamol between the 19th and 32nd week of pregnancy, the risk of behavioral problems increased by 46 percent.
Researchers at the Icahn School of Medicine at Mount Sinai reported in January 2018 that paracetamol use during pregnancy was linked to an increased rate of speech delay in girls. In an initial study of this type, researchers found an increased rate of speech delay in girls 30 months old who were born to mothers who frequently used acetaminophen in early pregnancy. These results are consistent with studies reporting decreased IQ and increased communication problems in children born to mothers who used more acetaminophen during pregnancy. Looking at language development is important because it has been shown to be predictive of other neurodevelopmental disorders in children. The Swedish Environmental Longitudinal, Mother and Child, Asthma and Allergy Study (SELMA) provided data for this research. Information was collected from 754 women enrolled in the study between weeks 8-13 of their pregnancy.
Although small amounts of paracetamol are excreted in breast milk, no undesirable effects on the infant have been reported when taking paracetamol while breastfeeding.
Method of application and dosage
Paracetamol can be administered orally , rectally, or intravenously . Paracetamol is dosed depending on age and body weight. For oral use, 10 to 15 mg of paracetamol per kg of body weight are generally used as a single dose (for example 1000 mg three times a day) and up to 60 mg / kg of body weight as the total daily dose. For adults (from 43 kg) this corresponds to a maximum daily dose of around 4000 mg divided into three to four individual doses. Dose adjustments are made in patients with impaired kidney or liver function.
Interactions
Probenecid inhibits the glucuronidation of paracetamol and thus its excretion. An inhibition of the excretion of paracetamol can also be observed after concomitant intake of salicylamide . Alcohol and drugs, as inducers of cytochrome P450 enzyme system function, such as carbamazepine and barbiturates , lead to an increased formation of liver harmful metabolic products ( metabolites ) of paracetamol. Ion exchangers such as cholestyramine reduce the absorption of paracetamol. The onset of the effect of paracetamol can be slowed down or accelerated by drugs that affect gastrointestinal activity, such as metoclopramide .
Paracetamol itself only rarely affects the effect of other drugs. When taken regularly, paracetamol increases the effects of anticoagulants such as phenprocoumon and warfarin . The side effects of zidovudine , which damage the blood count, can also be increased if paracetamol is taken at the same time. Furthermore, there has been increasing evidence recently that paracetamol and certain other pain relievers from the group of non-steroidal anti-inflammatory drugs or non-steroidal anti-inflammatory drugs (NSAIDs) , such as. B. acetylsalicylic acid (aspirin), can make vaccines less effective by the body making fewer of the protective antibodies after vaccination . The latter is attributed to the fact that drugs such as paracetamol impair the terminal differentiation of B cells into antibody-producing plasma cells . Researchers and doctors therefore advise avoiding appropriate medication for some time before and after vaccination.
Side effects
Paracetamol only rarely or very rarely shows undesirable effects when used as intended . None of the side effects related to acetaminophen are more common than 1 in 1,000 patients. These include an increase in certain liver enzymes ( transaminases ) in the serum (frequency: 0.01–0.1%). Very rarely (frequency: <0.01%) or in isolated cases serious changes in the blood count such as thrombocytopenia (reduced number of blood platelets) and agranulocytosis could be observed. Just as rarely, allergic reactions in the form of a simple skin rash or hives up to a shock reaction can occur. Also with a frequency of less than 0.01% there was a spasm of the respiratory muscles in sensitive persons ( analgesic asthma ). If paracetamol is used as directed, the risk of analgesic nephropathy is very low. Whether this risk is increased by combined use with acetylsalicylic acid and caffeine is a matter of controversy.
Epidemiological studies link paracetamol consumption in childhood, depending on the dose, with a long-term increased risk of asthma and an increased risk of inflammation of the nasal mucosa and the conjunctiva ( allergic rhinitis ) and skin inflammation ( eczema ). Other studies contradict the thesis that paracetamol promotes asthma.
The breakdown of paracetamol consumes glutathione and can lead to a deficiency of sulfur-containing amino acids in older patients, which in the long term can generally lead to cardiovascular susceptibility (susceptibility of the blood circulation) via the glutathione deficiency.
Long-term use of paracetamol is suspected to increase the risk of a number of blood cancers.
In view of the severe and life-threatening skin reactions observed in recent years with the use of paracetamol, the FDA advises users to consult their doctor if they develop skin reactions and to discontinue use of the preparation. A corresponding warning is to be printed on the drug packs of preparations containing paracetamol in the USA.
The Pharmacovigilance Committee for Risk Assessment (PRAC) of the European Medicines Agency (EMA) recommended in September 2017 that extended-release paracetamol products be withdrawn from the market. The risk of an overdose outweighs the advantage of a longer-acting preparation. A final decision by the EMA is still pending. The manufacturers concerned still have the opportunity to request a further review by the PRAC.
New studies suggest that acetaminophen affects compassion and empathy. It seems to decrease the ability to empathize with the sensation of pain.
Harmful use and overdose
Paracetamol overdoses as a result of ignorance of the maximum daily dose, non-observance of contraindications and restrictions on use as well as self-damaging, mostly suicidal intent are often associated with severe impairment of liver function. An overdose of more than 150 milligrams per kilogram of body weight, equivalent to 10 grams for adults, can lead to irreversible damage to the liver cells or even to liver failure . Alcoholics or patients with a reduced excretion of paracetamol can suffer liver damage even with a significantly lower dose. In England and Wales , around 30,000 patients per year are hospitalized with paracetamol poisoning as a result of suicidal intent, of whom around 150 succumb to poisoning. A restriction in the size of paracetamol preparations in the UK only showed a reduction in suicides after a few years. As in the UK, paracetamol is the most common cause of acute liver failure in the US, with around half of all intoxications occurring unintentionally. In 2011, the US FDA asked the manufacturers of drugs in which paracetamol is combined with an opioid to limit the paracetamol dose to 325 mg per dosage unit in order to reduce the risk of toxic effects on the liver. The FDA also wants to develop measures for OTC drugs .
The first symptoms of acute acetaminophen poisoning, which occur within the first 48 hours and peak after about four to six days, are nausea, vomiting, loss of appetite, paleness and persistent abdominal pain as signs of liver damage. At the same time, clinical values such as liver transaminases , lactate dehydrogenase , bilirubin values and prothrombin time can be increased. If no prompt treatment is followed, around 10% of patients with acute acetaminophen poisoning will suffer permanent, severe liver damage. In turn, around 10 to 20% of these patients die as a result of liver failure. Acute kidney failure occurs less frequently. In one study, nephropathy was observed in patients with previous renal impairment who took naproxen and paracetamol at the same time (cumulative 0.4 and 1.0 kg over years). This indicates a combined negative effect of naproxen and paracetamol. Other liver-independent symptoms observed after acetaminophen poisoning are heart muscle abnormalities and pancreatitis .
The cause of the liver toxicity of paracetamol is its metabolite N- acetyl- p -benzoquinone imine (NAPQI). The formation of this liver-damaging metabolite is increased by regular consumption of alcohol and drugs with an enzyme-inducing effect, such as carbamazepine. A suitable antidote for paracetamol poisoning is N -acetylcysteine , which scavenges toxic paracetamol metabolites, such as N -acetyl- p -benzoquinonimide, with the formation of non-toxic conjugates. This can prevent progression to irreversible liver damage or liver failure, provided the antidote is administered in good time. N -acetylcysteine is considered effective if given within ten hours. Various treatment regimens recommend the use of about 150 mg / kg body weight as a single dose and a total dose of 300 to 1330 mg / kg body weight divided over 20 to 68 hours. For this purpose, N-acetylcysteine is available for intravenous administration as well as for oral use ( effervescent tablets ). Activated charcoal can also be used immediately for up to about an hour after an overdose of paracetamol . In the case of advanced acute liver failure, liver transplantation is the only remaining treatment option that gives the patient a chance of survival.
Application in veterinary medicine
Because of its potentially damaging effects on the liver, paracetamol should be used with caution in veterinary medicine. In cats and young animals, the administration of paracetamol very quickly leads to poisoning with methaemoglobin formation , anemia , hemoglobinuria , liver damage, jaundice , dyspnoea and palpitations , as these can only insufficiently glucuronide the active ingredient . Dogs tolerate the active ingredient well, but the duration of action is very short (about two hours), so that the active ingredient is practically useless. The use of paracetamol in pigs is permitted among farm animals, whereby a residue limit is not necessary (Appendix II of Regulation 2377/90 ). Even the smallest amounts of paracetamol are said to have a lethal effect on snakes.
pharmacology
Mode of action (pharmacodynamics)
In contrast to the pain relievers acetylsalicylic acid or ibuprofen , paracetamol has an anti-inflammatory effect that can only be determined under laboratory conditions and is accordingly not included in the group of classic " non-steroidal anti-inflammatory drugs " (also: non-steroidal anti-inflammatory drugs, NSAIDs ; English non-steroidal anti-inflammatory drugs, NSAID ). In contrast to the classic NSAIDs, paracetamol has hardly any effect on the peripheral cyclooxygenase . For this reason, the side effects (including gastrointestinal ulcers) are much less pronounced. Paracetamol also has practically no influence on the aggregation of the blood platelets and therefore no anti-coagulant effect like acetylsalicylic acid.
The exact mechanism of action of paracetamol is still unknown. It is known that several controversially discussed mechanisms interact and that the pain-relieving effect occurs to a not inconsiderable extent in the brain and spinal cord .
Inhibition of cyclooxygenases
Based on the discoveries of John Vanes, it was long assumed that the analgesic effect of paracetamol is due to the cyclooxygenases , the enzymes involved in inflammatory reactions and the development of pain. The cyclooxygenase enzymes play a key role in the transmission of pain to the brain through the formation of pain and inflammation mediators from the group of prostaglandins . An inhibition of cyclooxygenases could explain the comparable analgesic potency of paracetamol and acidic non-opioid analgesics such as acetylsalicylic acid and ibuprofen, but not the largely lacking anti-inflammatory effectiveness and the equally largely missing gastrointestinal side effects of paracetamol. The reason for these differences was assumed to be a different distribution of paracetamol and acidic non-opioid analgesics in body tissue, whereby paracetamol is evenly distributed in the body and acidic non-opioid analgesics accumulate in the sense of drug targeting, for example in the stomach and in the inflamed tissue. Another possible explanation was found with the discovery of the cyclooxygenase isoenzyme COX-3 , a variant of COX-1 that occurs in particular in the cerebral cortex . However, this thesis was rejected a few years later because the COX-3 is actually just another splice variant of the COX-1 and is not sufficiently expressed to produce a biological effect. According to recent studies, a weakly suppressing effect on the cyclooxygenase isoenzyme COX-1 and a strong one on COX-2 in vivo are responsible for the effects of paracetamol. This matches its weak effect on platelets (blood platelets).
Interactions with the serotonin system
Further experimental data suggest that paracetamol mediates its effects by activating serotoninergic pain-relieving mechanisms. In particular, serotonin receptors of the 5-HT 3 type should play an important role. The pain-relieving effect is attributed to a projection of serotoninergic neurons into the spinal cord. This hypothesis about the mechanism of paracetamol shows analogies to the mode of action of opioids such as morphine . Alternatively, an analgesic serotoninergic effect of paracetamol can also be interpreted as a consequence of the inhibition of prostaglandin function, since most serotoninergic neurons also express prostanoid receptors.
Interactions with the endocannabinoid system
An interaction of paracetamol with the body's own cannabinoid system ( endocannabinoid system ) was suspected due to a weak euphoric , relaxing and calming effect of aniline-type analgesics in addition to the analgesic effect . Indeed, an interaction of paracetamol with the endocannabinoid system has been demonstrated in vivo . A metabolite of paracetamol, N- arachidonoylphenolamine , which is especially formed in the brain, has an antipyretic and analgesic effect via an indirect effect on cannabinoid receptors. N -Arachidonoylphenolamine interacts with the vanilloid receptor TRPV1, which occurs on many free nerve endings that function as nociceptors and is also involved in the regulation of body temperature. In addition, this paracetamol metabolite inhibits the cellular re- uptake of anandamide and thus leads to an increase in the concentration of this endogenous cannabinoid .
Other mechanisms of action
In addition to an interaction of paracetamol with cyclooxygenases, the serotonin system and the endocannabinoid system, an inhibiting influence of paracetamol on hyperalgesia caused by glutamate or substance P is discussed as the cause of its analgesic effect. Paracetamol is also involved in inhibiting the release of the messenger substance nitrogen monoxide (NO).
In addition, there are indications that paracetamol is not only able to alleviate physical pain, but also psychological suffering caused by social exclusion or rejection (so-called social pain ). According to the authors, this is an indication that there is a substantial overlap between physical and psychological pain with regard to the affected brain regions.
Pharmacokinetics
The maximum effective concentration of paracetamol is reached after about 30 to 60 minutes after oral administration. With rectal use, which achieves a bioavailability of 68 to 88%, maximum plasma concentrations are reached after approximately 3 to 4 hours. The plasma half-life is 1 to 4 hours. In premature babies it can be significantly higher due to a not yet fully developed metabolic system.

The decomposition of paracetamol is carried out mainly in the liver , where the majority of the substance as part of a Phase II reaction by combining with sulfate or glucuronic inactivated ( glucuronidation ) and then via the kidney is excreted.
The toxic effect can be traced back to a product that is produced in small quantities, which is produced in particular via the breakdown via the cytochrome P450 enzyme system , N- acetyl- p -benzoquinone imine . The cytochrome P450 isoenzyme CYP 2E1 , but also CYP 1A2 and CYP 3A4 , are particularly involved in the formation of this very reactive metabolite . Normally, N- acetyl- p -benzoquinone imine is immediately captured by the reaction with glutathione (GSH) and the resulting product is excreted via the kidneys. However, glutathione is only available to a limited extent in the liver and its replication cannot be increased sufficiently. Therefore, the glutathione portion is exhausted in the event of an acute paracetamol overdose. The N- acetyl- p -benzoquinone imine now reacts with structural and functional proteins of the liver cells , which can lead to liver cell necrosis and clinical liver failure. Chronic alcohol consumption and enzyme-inducing drugs increase the metabolism of paracetamol via the cytochrome P450 enzyme system to N -acetyl- p -benzoquinone imine and thus increase the toxicity of paracetamol.
An alternative route of degradation of paracetamol involving the cytochrome P450 isoenzymes CYP 2A6 and CYP 2B1 leads to 3-hydroxyparacetamol. This metabolite, which is excreted after glucuronidation, shows a significantly lower toxicity than N- acetyl- p -benzoquinone imine.
chemistry
structure
Paracetamol is a derivative of para - aminophenol , i.e. a phenol ( N -acetyl- p -aminophenol) and a derivative of aniline ( p -hydroxyacetanilide) at the same time . Paracetamol can also be understood as acetamide , i.e. as the amide of acetic acid , from which the name N - (4-hydroxyphenyl) acetamide, assigned according to IUPAC regulations, results.
Due to the aniline structure it contains, paracetamol as well as acetanilide , phenacetin and propacetamol belong to the analgesic group of aniline derivatives. Acetanilide, phenacetin and propacetamol can be viewed as precursors ( prodrugs ) that are converted to paracetamol in the organism.

|

|
|

|
| Acetanilide | Paracetamol | Phenacetin | Propacetamol |
Substance properties
Paracetamol is a white, crystalline solid that occurs in at least two different modifications. This polymorphism is of pharmaceutical importance and has an impact on the compressibility of the drug. Orthorhombic paracetamol shows a compressibility that is superior to the thermodynamically more stable monoclinic modification. Paracetamol is readily soluble in alcohols in both modifications , but only moderately in cold water (14 g / l at 25 ° C), but it is in boiling water. Paracetamol has a density of 1.293 grams per cubic centimeter. As a phenol, it is slightly acidic. The pH of a saturated, aqueous solution is around six. Paracetamol has a characteristic, slightly bitter taste .
Manufacturing
Various synthetic routes have been described for the production of paracetamol. The classic process uses the N -acetylation of p - aminophenol . This raw material can be produced by nitration and subsequent reduction of phenol or alternatively starting from aniline or p - chlorophenol . To acetylate the aminophenol, it is allowed to react with excess acetic anhydride, the end product as well as N , O -diacetylated by-product being formed with elimination of acetic acid . The latter hydrolyses in an aqueous medium or in a weakly alkalized medium due to the higher hydrolysis sensitivity of the esters - compared to the amide bond, selectively to paracetamol.
One process of industrial large-scale production starts with phenol and comprises three steps. Phenol is acetylated with acetic anhydride in the presence of hydrofluoric acid in the para position to form p -hydroxyacetophenone . Alternatively, p- hydroxyacetophenone can also be obtained from phenyl acetate at low temperature and with aluminum chloride as the Lewis acid ( Fries rearrangement ). The p -hydroxyacetophenone is then condensed with hydroxylamine to form the oxime . This rearranges in the presence of thionyl chloride according to Beckmann to paracetamol.
A more recent variant is the reducing amidation of p -nitrophenol with thioacetic acid .
Analytics
According to the European Pharmacopoeia , paracetamol can be identified with the help of chemical and instrumental analytical methods. Paracetamol can be detected by oxidation with potassium dichromate with the formation of a blue dye. The acetyl group can be detected after hydrolysis with the help of lanthanum nitrate and iodine . Alternatively, paracetamol can be detected by a positive marquis reaction after hydrolysis with the help of formaldehyde . The hydrolysis product also gives positive evidence for primary aromatic amines. The phenolic structure can also be identified with iron (III) chloride , which forms a blue, acid-labile complex.
The content of paracetamol is determined according to the European Pharmacopoeia after hydrolytic cleavage of paracetamol to p- aminophenol in the classic way as oxidimetric titration with the help of cerimetry . Alternative methods of determination of levels include instrumental methods such as HPLC . For the quantitative determination of paracetamol in urine, blood plasma or serum, colorimetric assays and immunassays are available in addition to HPLC and gas chromatography methods.
Given its frequent use, paracetamol can now also be detected in rivers in addition to ibuprofen .
Commercial preparations
Economic data, tax regulation
Paracetamol is one of the best-selling drugs worldwide. The monopreparation Paracetamol-ratiopharm was the second most frequently purchased drug in Germany with over 20 million packaging units in 2008. The combination preparation Thomapyrin (12.4 million packaging units in 2008) can also be found in the top 10 of the best-selling drugs. The total annual turnover of paracetamol in Germany is estimated at around 31 million packs with a market value of around 60 million euros.
Paracetamol preparations for oral administration for the treatment of mild to moderately severe pain and / or fever in a total amount of active ingredients of up to 10 g per package and for rectal use are not subject to prescription in Germany . Oral quantities of more than 10 g were made subject to the prescription requirement in April 2009 with the aim of reducing the frequency of paracetamol poisoning caused by improper use. Similar restrictions in the UK resulted in a small decrease in paracetamol-related deaths. An application for a general prescription requirement for paracetamol in Germany was rejected in 2012. In Germany, paracetamol is only available in rapid-release dosage forms - for countries such as Belgium, Denmark, Finland, Luxembourg, Portugal, Romania and Sweden, where sustained-release versions are also available, the Pharmacovigilance Committee of the European Medicines Agency confirmed its recommendation in December 2017, to suspend the approval of these dosage forms, the coordination group of the association of national approval authorities agreed.
A prescription is required for paracetamol-containing infusion solutions or for use in veterinary medicine, as well as for oral combination preparations with prescription substances such as codeine , metoclopramide and tramadol .
Monopreparations
Acetalgin (CH), ben-u-ron (D, A, CH), Captin (D), Contac (D), Contra-Pain P (CH), Dafalgan (CH), Dolprone (CH), Enelfa Dr. Henk (D), GRIPPEX (D), Mexalen (A), Panadol (CH), Parapaed (D), Perfalgan (A, D, CH), RubieMol (A), Tylenol (USA, CH) as well as numerous generics.
Combination preparations
- with acetylsalicylic acid : Fibrex (D), Thomapyrin 300 mg / 200 mg (D, A)
- with butylscopolamine : Buscopan Plus (A, D)
- with caffeine : Azure (D), COPYRKAL (D), Neopyrin (D), Octadon (D), Panadol Extra (CH), Prontopyrin (D), Vivimed (D)
- with codeine : Contraneural (D), Gelonida (D), Nedolon (D), Optipyrin (D), Paracetamol comp. STADA (D), talvosilen (D), Titretta (D), Co – Dafalgan (CH)
- with diphenhydramine : Panadol PM (USA)
- with metoclopramide : Migraeflux MCP (D), Migraine-Neuridal (D), Migräneerton (D), Migralave + MCP (D)
- with phenylephrine : Doregrippin (D)
- with Tramadol : DOLEVAR (D), Zaldiar (CH, D)
- with ascorbic acid (vitamin C): Mexa-Vit C (A)
Multiple combinations:
- with acetylsalicylic acid and caffeine: Chephapyrin (D), dolomo (D), Dolopyrin (D), HA tablets N (D), Melabon (D), Neuralgin (D), Novo Petrin (D), ratiopyrin (D), Thomapyrin CLASSIC (D), Thomapyrin INTENSIV (D), TITRALGAN (D), Thomapyrin (A), InfluASS (A), Irocophan (A)
- with ascorbic acid, caffeine and chlorphenamine : Grippostad (D)
- with caffeine and codeine: Azur compositum (D)
- with acetylsalicylic acid and ascorbic acid: Grippal + C (D)
- with guaifenesin , phenylephrine and ascorbic acid: WICK DayMed cold drink for the day (D)
- with phenylpropanolamine and dextromethorphan : Basoplex cold capsules (D), WICK DayMed cold capsules (D)
- with doxylamine , ephedrine , dextromethorphan: WICK MediNait cold syrup (D)
- with doxylamine and dextromethorphan: WICK MediNait cold syrup with honey and chamomile aroma (D, CH)
- with phenylephrine and dextromethorphan: Contac Cold Trunk Forte (D)
- with pheniramine , phenylephrine, ascorbic acid: NeoCitran (A, CH)
literature
- A. Bertolini, A. Ferrari, A. Ottani, S. Guerzoni, R. Tacchi, S. Leone: Paracetamol: new vistas of an old drug . In: CNS Drug Reviews . tape 12 , no. 3–4 , 2006, pp. 250–275 , doi : 10.1111 / j.1527-3458.2006.00250.x , PMID 17227290 .
- Ernst Mutschler u. a .: Mutschler - drug effects textbook of pharmacology and toxicology . 9th edition. Scientific Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 .
- RE Brandlistuen, E. Ystrom, I. Nulman, G. Koren, H. Nordeng: Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study . In: International Journal of Epidemiology . tape 42 , no. 6 , January 10, 2014, p. 1702-1713 , doi : 10.1093 / ije / dyt183 .
Web links
- Paracetamol supplements. Swiss Medicines Compendium
- Superbrands: Panadol . (PDF; English)
- Paracetamol entry at Vetpharm, accessed March 28, 2012.
Individual evidence
- ↑ a b c d e f g Entry on paracetamol in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b Entry on acetylaminophenols. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b B. Hinz, O. Cheremina, K. Brune: Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. In: FASEB J . 2008 Feb; 22 (2) pp. 383-390. PMID 17884974 .
- ↑ R. Picciochi, HP Diogo, ME Minas da Piedade: Thermo Chemistry of paracetamol. In: J. Therm. Anal. Calorim. 100, 2010, pp. 391-401. doi: 10.1007 / s10973-009-0634-y
- ↑ P. Di Martino, P. Conflant, M. Drache, JP Huvenne, AM Guyot-Hermann: Preparation and physical characterization of forms II and III of paracetamol. In: J. Therm. Anal. 48, 1997, pp. 447-458.
- ↑ a b c d Entry on paracetamol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ HN Morse: About a new method of representing acetylamidophenols . In: Reports of the German Chemical Society . tape 11 , no. 1 , 1878, p. 232–233 , doi : 10.1002 / cber.18780110151 ( bnf.fr [accessed January 3, 2016]).
- ^ J. Von Mering: Contributions to the knowledge of the Antipyretica. In: Ther. Monthly 7, 1893, pp. 577-587.
- ^ A Festival of Analgesics. Chemical Heritage Foundation, 2001.
- ↑ About Bene. Retrieved January 6, 2016 .
- ↑ US Mantzke, AM Brambrink: Paracetamol in childhood. Current state of knowledge and information for a rational use for postoperative analgesia . In: The anesthesiologist . Volume 51, Issue 9, September 2002, pp. 735-746 , doi : 10.1007 / s00101-002-0359-9 .
- ↑ G. Dunea: Death over the counter. In: Br Med J . 1983, 286, pp. 211-212. PMID 6401533 ; PMC 1546319 (free full text, PDF).
- ↑ KA Wolnik, FL Fricke, E. Bonnin, CM Gaston, RD Satzger: The Tylenol tampering incident - tracing the source. In: Anal. Chem. 56, 1984, pp. 466A-470A, 474A. PMID 6711821 .
- ↑ Ulla Thiede: Do we have a drug against Covid-19 soon? In: general-anzeiger-bonn.de. April 10, 2020, accessed April 13, 2020 .
- ↑ a b c d e f g h i j k ( page no longer available , search in web archives )
- ↑ Technical information: Fixed drug combination Paracetamol 400 mg / Caffeine 50 mg tablets Federal Institute for Drugs and Medical Devices . As of January 23, 2008.
- ↑ HC Diener, V. Pfaffenrath, L. Pageler, H. Peil, B. Aicher: The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study . In: Cephalalgia . tape 25 , no. 10 , October 2005, p. 776-787 , doi : 10.1111 / j.1468-2982.2005.00948.x , PMID 16162254 .
- ↑ S. Evers, A. May, G. Fritsche, P. Kropp, C. Lampl, V. Limmroth, V. Malzacher, S. Sandor, A. Straube, HC Diener: Acute Therapy and Prophylaxis of Migraine - Guideline of the German Migraine - and headache society and the German Society for Neurology . In: Neurology . tape 27 , no. 10 , 2008, p. 933-949 ( dmkg.de [PDF]).
- ↑ German Migraine and Headache Society: Treating tension headaches yourself - self-medication for patients (PDF; 349 kB).
- ↑ Technical information: Fixed drug combination paracetamol 500 mg / codeine phosphate hemihydrate 30 mg Federal Institute for Drugs and Medical Devices. As of September 5, 2008.
- ^ V. Persky, J. Piorkowski, E. Hernandez a. a .: Prenatal exposure to acetaminophen and respiratory symptoms in the first year of life . In: Ann Allergy Asthma Immunol . tape 101 , no. 3 , September 2008, p. 271-278 , doi : 10.1016 / S1081-1206 (10) 60492-9 , PMID 18814450 , PMC 2578844 (free full text).
- ^ H. Allmers, C. Skudlik, SM John: Acetaminophen use: a risk for asthma? In: Curr Allergy Asthma Rep . tape 9 , no. 2 , March 2009, p. 164-167 , PMID 19210907 .
- ↑ H. Farquhar, A. Stewart, E. Mitchell et al. a .: The role of paracetamol in the pathogenesis of asthma . In: Clin Exp Allergy . tape 40 , no. 1 , January 2010, p. 32–41 , doi : 10.1111 / j.1365-2222.2009.03378.x , PMID 20205695 .
- ↑ Bremer L .: Paracetamol Medication During Pregnancy: Insights on Intake Frequencies, Dosages and Effects on Hematopoietic Stem Cell Populations in Cord Blood From a Longitudinal Prospective Pregnancy Cohort; EBioMedicine, October 2017
- ↑ RE Brandlistuen, E. Ystrom, I. Nulman, G. Koren, H. Nordeng: Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study . In: Int J Epidemiol. tape 42 , p. 1702-1713 , PMID 24163279 .
- ↑ Paracetamol could cause behavior problems in children. Side effects of paracetamol. In: Der Spiegel. 43/2016, Retrieved October 23, 2016.
- ↑ C.-G. Bornehag et al .: Prenatal exposure to acetaminophen and children's language development at 30 months. In: European Psychiatry. June 1, 2018, accessed June 16, 2019 .
- ↑ Press Release: Acetaminophen Use During Pregnancy Associated With Elevated Rate of Language Delay in Girls, Mount Sinai Researchers Find. Mount Sinai, January 10, 2018, accessed June 16, 2019 .
- ↑ S. Bancos, MP Bernard, DJ Topham, RP Phipps: Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells . In: Cell Immunol . tape 258 , no. 1 , 2009, p. 18-28 , PMID 19345936 .
- ↑ VA Blaho et al. a .: Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17 . In: J Immunol . tape 183 , no. 9 , 2009, p. 5644-5653 , PMID 19843949 .
- ↑ R. Prymula et al. a .: Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomized controlled trials . In: The Lancet . tape 374 , no. 9698 , 2009, p. 1339-1350 , PMID 19837254 ( soniped.org [PDF]). soniped.org ( Memento from November 14, 2011 in the Internet Archive )
- ↑ Common Pain Relievers May Dilute Power of Flu Shots. University of Rochester Medical Center (URMC), accessed July 27, 2011 .
- ↑ Painkillers weaken vaccination protection. In: Spiegel online . Retrieved December 4, 2009 .
- ↑ MP Bernard, RP Phipp: inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation . In: Immunology . tape 129 , no. 1 , 2010, p. 87-96 , PMID 20050331 .
- ^ Over-the-Counter Pain Drugs May Affect Vaccine Strength. WXXI, accessed July 27, 2011 .
- ↑ The vaccination effect can be significantly weakened by some drugs. Pulmonologists online, accessed April 17, 2019 .
- ^ Effect of Prophylactic Paracetamol Administration at Time of Vaccination on Febrile Reactions and Antibody Responses in Children F1000 Ranking: "Exceptional" and Changes Clinical Practice. Medscape , accessed July 26, 2011 .
- ^ AR Feinstein, LA Heinemann, GC Curhan u. a .: Relationship between nonphenacetin combined analgesics and nephropathy: a review. Ad Hoc Committee of the International Study Group on Analgesics and Nephropathy . In: Kidney Int . tape 58 , no. 6 , December 2000, pp. 2259-2264 , doi : 10.1046 / j.1523-1755.2000.00410.x , PMID 11115060 .
- ↑ R. Beasley: Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6-7 years: analysis from Phase Three of the ISAAC program. In: The Lancet . 372 (9643), Sep 20, 2008, pp. 1039-1048. PMID 18805332 .
- ↑ RW Beasley et al. a .: Acetaminophen Use and Risk of Asthma, Rhinoconjunctivitis and Eczema in Adolescents: ISAAC Phase Three . In: Am. J. Respir. Crit. Care Med. 2010, doi : 10.1164 / rccm.201005-0757OC , PMID 20709817 .
- ↑ AJ Lowe, JB Carlin, CM Bennett, CS Hosking, KJ Allen, CF Robertson, C. Axelrad, MJ Abramson, DJ Hill, SC Dharmage: Paracetamol use in early life and asthma: prospective birth cohort study. In: BMJ (Clinical research ed.). Volume 341, 2010, p. C4616. PMID 20843914 , PMC 2939956 (free full text).
- ↑ G. Pickering, E. Schneider u. a .: Acetaminophen metabolism after major surgery: a greater challenge with increasing age. In: Clinical pharmacology and therapeutics . Volume 90, number 5, November 2011, pp. 707-711, doi: 10.1038 / clpt.2011.176 . PMID 21975347 .
- ↑ Roland B. Walter, Filippo Milano, Theodore M. Brasky, Emily White: Long-Term Use of Acetaminophen, Aspirin, and Other Nonsteroidal Anti-Inflammatory Drugs and Risk of Hematologic Malignancies: Results From the Prospective Vitamins and Lifestyle (VITAL) Study . In: Journal of Clinical Oncology . 2011, doi : 10.1200 / JCO.2011.34.6346 .
- ^ FDA Warns of Rare Acetaminophen Risk
- ↑ PRAC recommends modified-release paracetamol be removed from market , EMA summary of September 1, 2017, accessed on September 6, 2017
- ↑ Dominik Mischkowski, Jennifer Crocker, Baldwin M. Way: From Painkiller to Empathy Killer: Acetaminophen (Paracetamol) Reduces Empathy for Pain . In: Social Cognitive and Affective Neuroscience . 2016, p. nsw057 , doi : 10.1093 / scan / nsw057 ( oxfordjournals.org [accessed May 21, 2016]).
- ↑ a b A. M. Larson et al. a .: Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study . In: Hepatology . tape 42 , no. 6 , 2005, p. 1364-1372 , PMID 16317692 .
- ↑ a b c L. Jackson Roberts, Jason D. Morrow: Goodman & Gilman's the pharmacological basis of therapeutics . Eds: Alfred Gilman, Louis Sanford Goodman, Joel G. Hardman, Lee E. Limbird. McGraw-Hill, New York 2001, ISBN 0-07-112432-2 , Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout, p. 687-732 .
- ↑ Williams, Roger Lawrence; Jean-Pierre Benhamou; Lee, William Thomas: Acute liver failure . Cambridge University Press, Cambridge, UK 1997, ISBN 0-521-55381-4 .
- ↑ a b O. W. Morgan, C. Griffiths, A. Majeed: Interrupted time-series analysis of regulations to reduce paracetamol (acetaminophen) poisoning . In: PLoS Med . tape 4 , no. 4 , April 2007, pp. e105 , doi : 10.1371 / journal.pmed.0040105 , PMID 17407385 , PMC 1845154 (free full text).
- ↑ K. Hawton, H. Bergen, S. Simkin, S. Dodd, P. Pocock, W. Bernal, D. Gunnell, N. Kapur: Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyzes. In: BMJ. 346, 2013. doi: 10.1136 / bmj.f403
- ↑ a b c L. J. Chun et al. a .: Acetaminophen hepatotoxicity and acute liver failure. In: J Clin Gastroenterol. tape 43 , no. 4 , 2009, p. 342-349 , PMID 19169150 .
- ↑ FDA press release available as html , last accessed on January 14, 2011.
- ↑ J. Granese, K. Bright Bill, P. Osborne, CE Cox, LW Gaber: Analgesic nephropathy Selectively Affecting a unilateral non-functioning hypoplastic kidney . In: Clin. Nephrol. tape 68 , no. 2 , August 2007, p. 115-120 , PMID 17722712 .
- ↑ H. Kupferschmidt: Therapy of Paracetamol Poisoning Swiss Toxicological Information Center (PDF; 72 kB)
- ↑ Julia Nakagawa et al. a .: Side effects from non-approved non-steroidal anti-inflammatory drugs (NSAIDs) in 21 dogs. In: Small Animal Practice. 55, 2010, pp. 364-370.
- ↑ Wolfgang Löscher, Fritz Rupert Ungemach: Pharmacotherapy for pets and farm animals. 7th edition. Paul Parey, 2006, ISBN 3-8304-4160-6 , pp. 106-107.
- ↑ Silent killers in the service of environmental protection. US Army uses snake bait poisoned with paracetamol.
- ^ Stars and Stripes: Mice join fight against invasive snakes on Guam.
- ↑ RJ Flower, JR Vane: Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol) . In: Nature . tape 240 , no. 5381 , December 1972, p. 410-411 , PMID 4564318 .
- ↑ Olivier Boutaud, David M. Aronoff, Jacob H. Richardson, Lawrence J. Marnett, John A. Oates: Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H 2 synthases, In: Proc. Natl. Acad. Sci. USA . 2002, 99, pp. 7130-7135. PMID 12011469 ; PMC 124540 (free full text, PDF).
- ↑ K. Brune, KD Rainsford, A. Schweitzer: Biodistribution of mild analgesics . In: Br J Clin Pharmacol . 10 Suppl 2, October 1980, p. 279S-284S , PMID 6969084 , PMC 1430188 (free full text).
- ^ NV Chandrasekharan, H. Dai, KL Roos u. a .: COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic / antipyretic drugs: cloning, structure, and expression . In: Proc. Natl. Acad. Sci. USA band 99 , no. 21 , October 2002, p. 13926-13931 , doi : 10.1073 / pnas.162468699 , PMID 12242329 , PMC 129799 (free full text).
- ↑ Bela Kis, James A. Snipes, David W. Busija: Acetaminophen and the Cyclooxygenase-3 Puzzle: Sorting out Facts, Fictions, and Uncertainties . In: Journal of Pharmacology and Experimental Therapeutics . tape 315 , no. 1 , September 19, 2005, p. 1-7 , doi : 10.1124 / jpet.105.085431 .
- ↑ C. Frölich: Selective Cyclooxygenase Inhibitors: A New Generation of Antirheumatic Drugs. In: Deutsches Ärzteblatt . 93 (47), 1996, pp. A-3100 / B-2632 / C-2438 Medical Report.
- ↑ G. Pickering, V. Estève, MA Loriot, A. Eschalier, C. Dubray: Acetaminophen reinforces descending inhibitory pain pathways . In: Clin. Pharmacol. Ther. tape 84 , no. 1 , July 2008, p. 47-51 , doi : 10.1038 / sj.clpt.6100403 , PMID 17957182 .
- ↑ a b B. J. Anderson: Paracetamol (acetaminophen): mechanisms of action . In: Pediatr Anaesth . tape 18 , no. 10 , October 2008, p. 915-921 , doi : 10.1111 / j.1460-9592.2008.02764.x , PMID 18811827 .
- ↑ Mechanism of action of paracetamol , ncbi.nlm.nih.gov
- ^ A b Alfio Bertolini, Anna Ferrari, Alessandra Ottani, Simona Guerzoni, Raffaella Tacchi, Sheila Leone: Paracetamol: New vistas of an old drug. In: CNS Drug Reviews . 12, 2006, pp. 250-275. PMID 17227290 ; doi: 10.1111 / j.1527-3458.2006.00250.x .
- Jump up ↑ ED Högestätt, BA Jönsson, A. Ermund, DA Andersson, H. Björk, JP Alexander, BF Cravatt , AI Basbaum, PM Zygmunt: Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system, In: J. Biol. Chem. 280 (36), 2005, pp. 31405-31412. PMID 15987694 .
- ↑ CN Dewall, G. Macdonald, GD Webster, CL Masten, RF Baumeister, C. Powell, D. Combs, DR Schurtz, TF Stillman, DM Tice, NI Eisenberger: Acetaminophen reduces social pain: behavioral and neural evidence . In: Psychol Sci . tape 21 , no. 7 , 2010, p. 931-937 , PMID 20548058 .
- ↑ CN Dewall: Hurt feelings? You could take a pain reliever ... In: Harv Bus Rev . tape 89 , no. 4 , 2011, p. 28-29 , PMID 21510517 .
- ^ Red List 2009. Red List Service. ISBN 3-939192-30-9 .
- ↑ RA van Lingen, JT Deinum, JM Quak u. a .: Pharmacokinetics and metabolism of rectally administered paracetamol in preterm neonates . In: Arch. Dis. Child. Fetal Neonatal Ed. tape 80 , no. 1 , January 1999, p. F59-F63 , PMID 10325815 , PMC 1720876 (free full text).
- ↑ Charge density and electrostatic potential analyzes in paracetamol. In: Acta Cryst. B65, 2009, pp. 363-374.
- ↑ Stephen PF Miller, Andre S. Raw, Lawrence X. Yu: Polymorphism: in the Pharmaceutical Industry . Ed .: Rolf Hilfiker. John Wiley & Sons, Chichester 2006, ISBN 3-527-31146-7 , Scientific considerations of pharmaceutical solid polymorphism in regulatory applications, pp. 385-404 .
- ^ A b Thomas Christoph, Helmut Buschmann: Analgesics: From Chemistry and Pharmacology to Clinical Application . Ed .: Bernd Sundermann, Helmut Buschmann, Thomas Christoph, Elmar Friderichs, Corinna Maul. Wiley-VCH, Weinheim 2002, ISBN 3-527-30403-7 , Cyclogenase inhibition: From NSAIDs to selective COX-2 inhibitors, p. 13-126 .
- ↑ A. Bhattacharya et al. a .: Eco-friendly reductive acetamidation of arylnitro compounds by thioacetate anion through in situ catalytic regeneration: application in the synthesis of acetaminophen . In: Tetrahedron Letters . tape 47 , no. 19 , 2006, pp. 3221-3223 , doi : 10.1016 / j.tetlet.2006.03.057 .
- ↑ a b European Pharmacopoeia. 6th edition. Basic work 2008. Monograph Paracetamol. German pharmacist publishing house Stuttgart. ISBN 978-3-7692-3962-1 .
- ↑ Lee J, Park J, Go A, Moon H, Kim S, Jung S, Jeong W, Chung H: Urine Multi-drug Screening with GC-MS or LC-MS-MS Using SALLE-hybrid PPT / SPE. , J Anal Toxicol. 2018 Nov 1; 42 (9): 617-624, PMID 29762685
- ↑ Lu W, Zhao S, Gong M, Sun L, Ding L: Simultaneous determination of acetaminophen and oxycodone in human plasma by LC-MS / MS and its application to a pharmacokinetic study. , J Pharm Anal. 2018 Jun; 8 (3): 160-167, PMID 29922484
- ↑ Borrull J, Colom A, Fabregas J, Pocurull E, Borrull F: A simple, fast method for the analysis of 20 contaminants of emerging concern in river water using large-volume direct injection liquid chromatography-tandem mass spectrometry. , Anal Bioanal Chem. 2019 Mar; 411 (8): 1601-1610, PMID 30680425
- ↑ G. Glaeske, C. Schicktanz, K. Janhsen: GEK-Arzneimittelreport 2009. Asgard Verlag, 2009, ISBN 978-3-537-44068-6 .
- ↑ Ibuprofen three times as often as ASS In: APOTHEKE ADHOC September 28, 2014.
- ↑ ABDA press release of March 10, 2009 - Painkillers with paracetamol from April partially prescription-only ( Memento from January 2, 2016 in the Internet Archive )
- ↑ Apotheke Adhoc: Paracetamol remains OTC product. June 27, 2012, archived from the original on September 23, 2015 ; Retrieved November 10, 2014 .
- ↑ Julia Borsch: EMA recommends Aus for retarded paracetamol . In: DAZ.online . September 4, 2017 ( deutsche-apotheker-zeitung.de [accessed September 4, 2017]).
- ↑ Paracetamol-containing drugs with modified release: Measures to minimize risk and reduce damage in the event of overdose , BfArM, December 15, 2017.
- ↑ Contra Schmerz® P . April 23, 2018. Retrieved July 27, 2018.