acetic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | acetic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 4 O 2 | |||||||||||||||||||||
| Brief description |
pungent smelling liquid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 60.05 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.05 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
17 ° C |
|||||||||||||||||||||
| boiling point |
118 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
4.76 |
|||||||||||||||||||||
| solubility |
completely miscible with water (20 ° C) |
|||||||||||||||||||||
| Dipole moment | ||||||||||||||||||||||
| Refractive index |
1.3720 (20 ° C, λ = 589.3 nm) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
DFG / Switzerland: 10 ml m −3 or 25 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Acetic acid ( IUPAC ) (systematically ethanoic acid , Latin acidum aceticum ) is a colorless, caustic, hygroscopic , flammable liquid from the group of carboxylic acids . Acetic acid ( CH 3 –COOH ) has a characteristic sour taste and smell. It is a weak acid that only partially dissociates in aqueous solution .
Acetic acid is an important industrial chemical for the production of polymers such as polyvinyl acetate or cellulose acetate . The global demand in 2014 was around ten million tonnes per year. Acetic acid for industrial purposes is mostly obtained by the carbonylation of methanol or by the oxidation of acetaldehyde .
The acetic acid used in the household, called vinegar in a dilute aqueous solution , is obtained exclusively through the acetic acid fermentation of ethanol . As a food additive , it has the E number E 260. In addition to its use as a food, diluted acetic acid is used as a decalcifying agent.
nomenclature
The International Union of Pure and Applied Chemistry ( IUPAC ), an institution that issues recommendations on the nomenclature and terminology of chemical compounds, prefers the common name acetic acid as the standard name. At the same time, the IUPAC has defined the name ethanoic acid as a systematic name that results from the substitutive nomenclature .
The English part of the name acetic , the German name of the acetic acid anion, acetate , and the outdated names acetylic acid and acetoxylic acid are derived from the Latin word for vinegar, acetum . In the chemical abbreviations AcOH or HAc, Ac stands for the acetyl group or the acetoxy group , OH stands for the OH group of the carboxy group and H for the proton of the acid.
The term glacial acetic acid refers to the property of anhydrous acetic acid to freeze into ice-like crystals at a temperature of 16.6 ° C or lower . The name wood vinegar refers to acetic acid obtained from the dry distillation of wood. The names methyl formic acid , methane carboxylic acid and methyl carboxylic acid come from older substitutive nomenclatures.
history
European and Asian cuisine have used vinegar as a condiment for acidifying and preserving food for many centuries . The use as an ingredient in cosmetic products in ancient Egypt has also been proven. In Europe, its use as a food goes back to ancient times . Posca , a drink made from vinegar water, was a non-alcoholic drink in the Roman Empire . The antibacterial vinegar allowed the consumption of possibly microbiologically contaminated water. The chemical use of acetic acid was already known at that time. In the third century BC, the Greek philosopher and naturalist Theophrastus von Eresos described the action of vinegar on lead to produce white lead , a white pigment that was important in ancient times . At the end of the 18th and beginning of the 19th century, the opinion prevailed that acetic acid was the only vegetable acid and that all others consisted of their composite forms. Carl Wilhelm Scheele refuted this in 1786 by isolating gallic acid . However, it took a long time for this knowledge to become generally accepted. In 1814, Jöns Jakob Berzelius determined the composition of acetic acid. In the 18th century the application was of " Pestessig " or "Four Thieves Vinegar", a herbal extract vinegar-based, as protection against infectious diseases. Before coming into contact with the sick, it should be used to rinse the mouth and nose and wash the hands.
Fermentation of wine

As with the well-known aceto balsamico in the Italian region of Modena , the manufacturers traditionally obtained the vinegar from wine that was left open and fermented in the process . The Orléans process (open fermentation), developed in France in the Middle Ages , won the vinegar from wine, which was filled into large, shallow vats and placed open. Draft flies , also known as fruit or vinegar flies, brought in acetic acid bacteria that formed a skin on the surface of the wine, the so-called mother vinegar . This process is still used today to produce high-quality wine vinegars.
A further development took place in the 19th century with the Schüzenbach process, also known as the rapid vinegar or bondage process, and the round pumping process with the first surface fermenters . A further development was the Großraumbildnerverfahren . In the tie-up, generator or chip-forming process, the wine or alcohol-containing solution was trickled through large wooden generators, which were filled with beech shavings, for example , and served as a natural carrier for the colonization of bacteria. The air circulation , driven by the heat of reaction , ensured the supply of oxygen via a ventilation at the bottom of the container. Similar processes for the production of vinegar are still in use today.
The French scientist Louis Pasteur discovered the role of bacteria in the production of vinegar in 1856 . In 1868 he worked out selective growth conditions for the acetic acid bacteria for the first time and used them. In doing so, he laid the foundation for the controlled production of vinegar in the form of wine vinegar with an acetic acid content of around 6%. The process and the yield did not improve until 1949 with the introduction of a submerged process in the form of the "Frings Acetator", named after the company Heinrich Frings GmbH & Co KG in Bonn , which was significantly involved in the development. The submerged process is the most common form of production for biogenic acetic acid.
Dry distillation of wood

The production of acetic acid from wood vinegar according to Lowitz began around 1800 . Beech wood provided about 6% of the dry mass of acetic acid. The wood vinegar, which was cheaper to produce than the vinegar obtained by fermentation, reacted with lime to form calcium acetate , gray lime . A high concentration of acetic acid could be obtained from this by reaction with mineral acids, which was further concentrated by distillation . At the beginning of the 20th century, German industry produced around 35,000 tons of acetic acid per year using this process.
Industrial manufacture
The German chemist Hermann Kolbe succeeded in synthesizing acetic acid from inorganic compounds in 1845 . The photochemical reaction of tetrachlorethylene in the presence of water and chlorine led to trichloroacetic acid , a strong acid (p K S value: 0.65) which could be converted into acetic acid with sodium amalgam . However, the reaction found no technical application.
The first large-scale preparation was carried out in the first Wacker process by the hydration of acetylene under mercury sulfate / sulfuric acid - catalysis to acetaldehyde . This was further oxidized to acetic acid under manganese catalysis . The Wacker Chemie developed this method 1913th
After large quantities of ethylene were available in the 1960s, the Wacker-Hoechst process replaced the first Wacker process. The acetaldehyde is formed by the oxidation of ethylene . The installed production capacity in the 1970s was around 2.6 million tons per year.
With the expansion of petroleum processing , large quantities of gaseous hydrocarbons were produced in the refineries , which were initially neither used as fuels nor in the chemical industry. The butane and butenes produced were used on an industrial scale from 1952 in butane oxidation, which had been known since 1884. Acetic, formic , propionic and butyric acid as well as neutral products such as ketones , aldehydes , esters and alcohols were produced .
In 1941, the BASF chemist Walter Reppe demonstrated the effectiveness of carbonyls as catalysts for the production of carbonyl compounds. Based on this work, BASF developed a process with which methanol and carbon monoxide were converted into acetic acid under high pressure and temperatures . The methanol itself was a raw material that was not primarily based on petroleum, but was obtained from various raw material sources such as natural gas and coal using synthesis gas. In 1960 the BASF process was first implemented on a large scale in a plant in Ludwigshafen am Rhein . BASF continuously increased its capacity from initially 3600 metric tons per year to 45,000 metric tons per year in 1981. In 1966, the American Borden Chemical Co. built another plant based on the BASF process with a capacity of 45,000 metric tons per year in Geismar , Louisiana 64,000 t / a was increased.
In the late 1960s, Monsanto developed the Monsanto process , in which acetic acid is made by carbonylating methanol with carbon monoxide . In 1970, Monsanto built the first facility in Texas City with a starting capacity of 135,000 metric tons per year, which was increased to 270,000 metric tons per year by 1975. Shortly after this start, the BASF process became less economical and could no longer compete. In 1978, Celanese built the Clear Lake Plant in Seabrook , Texas, based on the Monsanto process, with a starting capacity of 27,000 metric tons per year. Process improvements increased the capacity to 900,000 tons per year.
In 1986, BP chemicals bought the rights to the Monsanto process without the modifications from Celanese and modified it with an iridium catalyst. This approach, known as the Cativa process, was further developed in the early 1990s to replace and improve the process at the Monsanto factory in Texas City .
Occurrence and biological importance
Free acetic acid
Acetic acid is a component of vegetable juices and essential oils . Alcoholic beverages that are exposed to the air for long periods of time form acetic acid due to the oxidation of the ethanol. Acetic acid bacteria, which are widespread in the environment, occur almost everywhere where yeasts ferment glucose or other sugars into ethanol. The bacteria further oxidize the resulting ethanol to acetic acid. In the intestinal tract of insects that feed on carbohydrates, acetic acid bacteria form part of the intestinal flora. The assimilation of acetic acid supplements the bee nutrition if necessary. The production of acetic acid by bacteria occurs as an undesirable side reaction in the production of silages such as corn silage ; too high a proportion of acetic acid in the silage makes it no longer beneficial for the cattle.
Acetobacter aceti , a Gram-negative bacterium, and the bacterium Clostridium acetobutylicum excrete acetic acid as part of their metabolism. These microorganisms occur wherever ethanol occurs as part of sugar fermentation. Acetobacter aceti grows best at temperatures of 25 to 30 ° C and a pH range of 5.4 to 6.3. Acetic acid is also found in vaginal lubrication in humans and other primates, where it serves as a mild antibacterial agent.
During the propionic acid fermentation for the maturation of hard cheese, streptococci and lactic acid bacteria ferment lactose into lactic acid ; in the further course propionic acid bacteria convert the lactic acid into acetic acid and propionic acid , which are components of the cheese aroma.
Part of the global methane production comes from the acetate metabolism of archaea like Methanosarcina thermophila . In the fermentation route, acetic acid is decarboxylated to methane and carbon dioxide :
Organic acids such as formic and acetic acid are components of the global troposphere and contribute to the acidification of precipitation. Acetic acid enters the atmosphere in forest fires, for example. Formic and acetic acids represent about a quarter of atmospheric non- methane - hydrocarbons . In addition to emissions from biomass , photochemical reactions contribute to the formation of acetic acid in the atmosphere.
Interstellar occurrence of acetic acid was first discovered in 1996 in the molecular cloud of Sagittarius B2 North, called the home of large molecules , about 390 light years from the center of the Milky Way and about 25,000 light years from Earth. Under laboratory conditions it has been proven that carbon dioxide and methane react to acetic acid via a radical mechanism already at 12 K under the influence of high-energy radiation .
This mechanism could explain the formation of the interstellar deposits.
Acetic acid salts
The naturally occurring but very rare minerals such as hoganite [Cu (CH 3 COO) 2 · H 2 O] or paceite [CaCu (CH 3 COO) 4 · 6 H 2 O] are examples of acetate occurrences in inanimate nature. The minerals were probably formed by the reaction of ores with acetic acid of vegetable origin. Another representative of the acetate minerals is calclacite [Ca (CH 3 COO) Cl · 5 H 2 O]. This is caused by the reaction of calcium-containing material with acetic acid, which was released from plant material such as wood.
Organic acetic acid compounds
Acetylated compounds , i.e. the replacement of a hydrogen atom by the acetyl group of acetic acid on the functional groups −OH, −SH and −NH 2 , but also directly on a −C − H bond, are widespread in nature and have a wide range of functions .
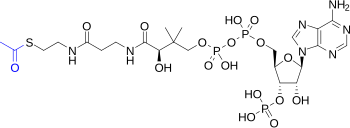
- Acetyl-Coenzyme A (Acetyl-CoA), the acetyl group bound to Coenzyme A as a thioester , is central to the metabolism of carbohydrates and fats and essential for the synthesis and oxidation of fatty acids and the oxidation of pyruvate in the citric acid cycle . The thioester bond is a very reactive bond, the hydrolysis is exergonic at −31.5 kJ / mol . Konrad Bloch and Feodor Lynen , who succeeded in isolating activated acetic acid, acetyl-coenzyme A, from yeast cells in 1951, received the Nobel Prize for Physiology or Medicine in 1964 for their discoveries that linked acetyl-CoA and fatty acid metabolism .
- Acetylcholine , an ester of acetic acid and the amino alcohol choline , is one of the most important neurotransmitters in many organisms, including humans. It plays an important role in learning and memory processes.
- The synthetic acetylsalicylic acid (brand name: aspirin) and the synthetic acetylcysteine are used as drugs.
In the case of biopolymers , acetylation modifies the polymer properties. Peracetylated polymers have a very low solubility:
- Chitin (acetylglucosamine) is the most widespread amino sugar polymer in nature . In fungi it is the main component of the cell wall and in arthropods it is the main component of the exoskeleton.
Acetylation is - after phosphorylation - the second most common selective, post-translational modification in eukaryotic cells. Acylation (or subsequent deacetylation) creates biologically differently active populations:
- N-acetylation of lysine in histones reduces the positive surface charges of these proteins and thus also their binding to DNA .
- N-terminal acetylation of proteins plays an important role in the synthesis, stability and localization of proteins.
Manufacturing
Worldwide production capacities for acetic acid are around 7 million tons per year. Between 1998 and 2006 there was an average worldwide production growth of 3% to 4% per year, with about 70% of annual production in the USA (1996: 36%; 2006: 32%), Western Europe (1996: 24%; 2006 : 17%) and Japan (1996: 16%; 2006: 11%). In comparison to these regions, East Asian production rose by 14% in 1996 to around 18% in 2006. The catalytic oxidation of light gasoline and the rectification of wood vinegar are only rarely used. Around 190,000 tons are produced annually worldwide by fermentation, with around 70% of the world demand for table vinegar being produced in around 700 bioreactors using the submerged process.
Biotechnical manufacturing
The biotechnical, fermentative production of acetic acid is the oxidation ("respiration") of ethanol by bacteria of the genera Acetobacter and Gluconobacter . From a biochemical point of view, it is a partial oxidation and not, as has been erroneously described, a form of fermentation . The bacteria convert ethanol produced by fermentation processes into acetic acid through a “subterminal oxidation” via acetaldehyde.
The oxidation takes place by membrane-associated alcohol dehydrogenases (ADH) and aldehyde dehydrogenases (ALDH), which contain pyrroloquinoline quinone (PQQ) as a prosthetic group and, in ADH, also heme c . The electrons released during the oxidation are transferred via ubiquinone to a membrane-bound oxidase .
The starting materials for the formation of acetic acid can be wine , beer or malt . The bacteria are dependent on a sufficient supply of oxygen and are very sensitive to oxygen-poor conditions. Even if the oxygen supply is interrupted for a few minutes, there is a significant decrease in ethanol oxidation. If ethanol is not available as a substrate , the acetic acid decomposes oxidatively to form carbon dioxide and water. The bacteria break down carbohydrates both via glycolysis and via the Entner-Doudoroff route to pyruvate , which is further metabolized in the citric acid cycle .
Some types of anaerobic bacteria, such as some of the genus Clostridium , can convert sugar directly into acetic acid without the intermediate stage ethanol using the following chemical reaction equation:
However, due to the lack of acid resistance of the bacteria, the concentration of the acetic acid produced in this way is below the concentration of ethanol-metabolizing strains and makes an enrichment by distillation necessary. The acetic acid fermentation of ethanol is therefore the more cost-effective form of production.
Butane and butene oxidation
The C4 hydrocarbons butane, 1-butene and 2-butene , which are produced in various refinery processes, were initially neither used as fuel nor as a raw material for the chemical industry. It has long been known that butane can be oxidized to acetic acid. Various companies such as the chemical works in Hüls developed technical processes for butane and butene oxidation. From the early 1950s, plants for the liquid phase oxidation of n- butane at around 170 to 200 ° C and 60 to 80 bar were built using the Hüls-butane process; an analogous process is based on butene .
The Celanese n Butane LPO process works at 54 bar and 175 ° C with cobalt acetate as the catalyst.
The accumulation of by-products such as other low molecular weight acids, ketones and other oxidation products made working up the reaction mixture difficult. The complex processing and alternative uses for the C4 cut made the operation of these systems uneconomical.
Wacker-Hoechst process
Large amounts of acetic acid are produced by the Wacker-Hoechst process via the oxidation of ethylene . The oxidation of ethylene in the presence of palladium (II) chloride as a catalyst produces acetaldehyde. The oxygen in the oxidation reaction comes from the water used as the solvent. The oxygen used in the process is used to reoxidize the catalyst using copper (II) chloride . The following balance equations summarize the catalytic cycle:
with the overall balance:
- .
The partial reactions (a) to (c) can be represented as coupled reactions:
The acetaldehyde formed as an intermediate product is oxidized to acetic acid by oxidation with air or oxygen using manganese (II) acetate as a catalyst. In an intermediate stage, peracetic acid is produced , which is reduced to acetic acid by the catalyst.
An older process obtained acetaldehyde from ethylene via acid-catalyzed hydration to form ethanol , which was dehydrated to acetaldehyde at higher temperatures in the Lebedew process .
Monsanto and Cativa process
Newer plants for the industrial synthesis of acetic acid work with the catalytic conversion of methanol with carbon monoxide under a pressure of 30 to 60 bar and at temperatures of 150 to 200 ° C in the Monsanto process .
The process uses a rhodium catalyst and has a selectivity of over 99% based on methanol. The anionic complex cis- [Rh (CO) 2 I 2 ] - is considered the active catalyst species . The process is an example of a homogeneous catalytic process and consists of several partial reactions.
The water-gas shift reaction is catalyzed as a side reaction , producing small amounts of carbon dioxide and hydrogen. Furthermore, propionic acid is produced through the carbonylation of ethanol, which enters the process as an impurity in the methanol .
BP Chemicals developed a variant of the process in 1966. Using an iridium (III) iodide catalyst precursor in the Cativa process, it was possible to achieve higher sales and lower capital expenditure in the construction of new plants; the iridium complex [Ir (CO) 2 I 2 ] - is considered to be the active species . In 2000, the first plant using this process was put into operation in Malaysia .
properties
Molecular Properties
The bond length of the carbon-carbon bond is 154 pm , that of the carbon-oxygen double bond 124 pm, that of the carbon-oxygen single bond 129 pm and that of the intermolecular hydrogen bond 261 pm.
The bond angles of the carboxy group are 120 °, the carbon-oxygen single bond having a partial π character . The bond of the oxygen atom of the hydroxyl group to the carboxy carbon atom occurs via sp 2 orbitals . The structure can be represented by two mesomeric boundary structures with a negative partial charge on an oxygen atom and a positive one on the partially double-bonded oxygen of the hydroxyl group.
Physical Properties
Acetic acid crystallizes in the orthorhombic space group Pna 2 1 (space group no.33 ) with the lattice parameters a = (1332 ± 2) pm , b = (408 ± 1) pm and c = (577 ± 1) pm. The molecules are linked by hydrogen bonds to form endless chains.
At 118 ° C, acetic acid has a relatively high boiling point compared to polar substances with roughly the same molar mass; for example, the boiling point of 1-propanol is 97 ° C. The reason for this is the ability of the acetic acid molecules to form hydrogen bonds via their carboxy groups. In the liquid phase, the acetic acid molecules form chain structures. In the gas phase, the dimer, made up of two acetic acid molecules that behave like a molecule with double molar mass, is the most stable form. The breaking of the chain structures and the transition of the dimers into the gas phase require a higher energy expenditure, recognizable by the "increased" Boiling temperature.
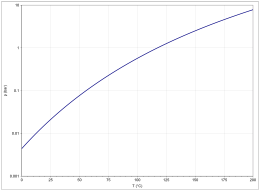
The vapor pressure function results from the Antoine equation according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.68206, B = 1642.540 and C. = −39.764 in the temperature range from 290.26 to 391.01 K.
The temperature dependence of the evaporation enthalpy can be calculated according to the equation Δ V H 0 = A exp (−αT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature ) with A = 22.84 kJ / mol, α = 0.0184, β = −0.0454 and T c = 592.7 K in the temperature range between 298 and 392 K.
As a potential electrolyte , pure acetic acid has a very low conductivity for electrical current, based only on autoprotolysis . The conductivity of pure acetic acid at 25 ° C is 6 · 10 −7 S · m −1 . Only when water is added does dissociation and the increase in conductivity occur. Anhydrous acetic acid solidifies to ice-like crystals at just 16.6 ° C.
| property | Type | Value and unity | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−484.5 kJ mol −1 −433 kJ mol −1 |
|
| Standard entropy | S 0 l, 1 bar S 0 g |
158.0 J mol −1 K −1 282.84 J mol −1 K −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −875.16 kJ mol −1 | |
| Heat capacity | c p | 123.1 J mol −1 K −1 (25 ° C) 2.05 J g −1 K −1 (25 ° C) 63.44 J mol −1 K −1 (25 ° C ) 1.06 J g −1 K −1 (25 ° C) |
as a liquid as a gas |
| Critical temperature | T c | 318.8 ° C | |
| Critical pressure | p c | 57.86 bar | |
| Acentric factor | ω c | 0.46652 | |
| Enthalpy of fusion | Δ f H 0 | 11.72 kJ mol −1 | at the melting point |
| Entropy of fusion | Δ f S 0 | 40.5 kJ mol −1 | at the melting point |
| Enthalpy of evaporation | Δ V H 0 Δ V H |
51.6 kJ mol −1 23.7 kJ mol −1 |
at normal pressure boiling point |
Chemical properties
Liquid acetic acid is a polar, hydrophilic and protic solvent . The dielectric constant ε is 6.2 (at 25 ° C). It mixes easily with polar and non-polar solvents such as water, chloroform and hexane . Acetic acid dissolves both polar compounds such as inorganic salts and sugars, and non-polar compounds such as low molecular weight alkanes. Acetic acid is no longer completely miscible with higher alkanes such as octane ; the miscibility decreases with increasing chain length of the alkanes.
In aqueous solution acetic acid reacts as a moderately strong acid ; the pKa value is 4.76. In a protolytic reaction , an equilibrium is established between the acetic acid and the acetate ion, which is strongly on the side of the acid. As with all carboxylic acids , the carboxylate group of the acetate ion is stabilized by mesomerism , which contributes significantly to the acidic reaction of the carboxylic acids:
The degree of dissociation of the acid in dilute solutions is only in the range of a few percent. In a 1 molar solution it is only about 0.5%. The resulting oxonium ion (H 3 O + ) leads to an acidic solution ( pH <7). With a 30% solution, corresponding to a 5 molar solution, the pH is 1.7, with a 40% solution 1.53 and with a 50% solution 1.31.

If the pH value of an acetic acid solution is increased by adding a strong base or adding acetates , a buffer solution is formed. If the pH value of the solution is the same as the pKa value of acetic acid, acetic acid and acetate ion are present in the same concentration. This is the optimal point of an acetic acid acetate buffer , at which the change in pH value when acids or bases are added is maximally buffered. This buffer system, which is effective in acid, is important for biochemical systems, since it has a favorable pKa value and the components involved do not negatively affect most organisms and biomolecules . It is a stable buffer system, which means that the conjugated acid-base pair remains in solution and cannot escape from the system as with the hydrogen carbonate buffer .
Acetic acid oxidizes completely in the air with the development of heat to form water and carbon dioxide . However, this happens extremely slowly at room temperature .
The salts of acetic acid are called acetates . They are mostly crystalline salts that contain the acetate anion (CH 3 COO - ) in their crystal lattices ( ion lattices ) . Base metals such as magnesium , calcium , zinc or iron dissolve in dilute acetic acid, forming water-soluble acetates and releasing hydrogen . In the presence of oxygen, the acetic acid reacts with copper to form copper acetate , a green, harmful salt that is better known as "verdigris". Acetic acid is used in diluted form to dissolve lime according to the following reaction equation:
Acetic acid reacts with ethanol acid catalyzed to form ethyl acetate , a widely used solvent . If 1-pentanol is used instead of ethanol , the result is amyl acetate , a strong-smelling compound. Acetic acid reacts with glycerine to form triacetin , which is used as a plasticizer for paints and adhesives. Allyl acetate is used as a fragrance .
Acetic acid reacts at 800 ° C with dehydration to form ketene . This in turn reacts with excess acetic acid to form acetic anhydride .
With thionyl chloride , acetic acid can be converted into acetyl chloride , which is used for esterification reactions. The chlorination leads to chloroacetic acid , which is used in the production of carboxymethyl celluloses , mercaptoacetic acid , crop protection agents , dyes or pharmaceuticals . With ammonia , ammonium acetate is initially formed , which is converted into acetamide by heating .
use
nutrition
Acetic acid is very important as a flavoring substance . Acetic acid ( E 260 ) and its salts potassium acetate (E 261), sodium acetate (E 262) and calcium acetate (E 263) are used as acidifiers for fruit and vegetables in cans and glasses (0.5–3% acetic acid) or as dough acidifiers . The acetic acid in sourdough is formed by heterofermentative sourdough bacteria . In addition, acetic acid is used in all variations of fish, canned foods, various marinades , delicatessen salads, mayonnaises and salad dressings together with sorbic acid (E 200) or benzoic acid (E 210). Pickled vegetables are vegetables that can be preserved with vinegar, among other things.
Various dairy products are also made using acetic acid. Mascarpone is made from cream that is thickened with acetic acid. Likewise, Ziger , a whey cheese made from whey by precipitating residual protein with acetic acid. Lead acetate , also known as lead sugar, was used as a sugar substitute to sweeten wine until modern times; the toxicity of lead sugar was unknown for a long time. The smell of wine like vinegar, the so-called vinegar tinge, is considered a wine defect .
The placing and washing of fresh meat is also done with the help of acetic acid. The bactericidal effect of acetic acid is that physiological processes can be suppressed by the decreased pH, and proteins denature . Household vinegar consists of biogenic vinegar and contains 5% acetic acid.
| Commercial varieties of vinegar | concentration |
|---|---|
| Table or table vinegar | 3.5-5% |
| Pickled vinegar | 5% |
| Wine vinegar | 6% |
| Double vinegar | 7% |
| Triple vinegar or spirit vinegar | 10.5% |
| Vinegar essence | 25% |
Vinegar essence is a 25% acetic acid solution in water , has a strong pungent smell and may only be used diluted in food. Vinegar essence is often used as an organic household cleaner. Aqueous solutions of acetic acid with an acid content greater than 15.5% may officially no longer be designated as vinegar.
Follow-on products
Acetic acid produced on an industrial scale is used almost exclusively for material use. More than 65% of world production is used for polymers based on vinyl acetate (43%) and cellulose acetate (25%). Vinyl acetate is the basis for polyvinyl acetate (PVAc), which is used in paints and adhesives, to a lesser extent in vinyl acetate copolymers such as ethylene vinyl acetates and polyvinyl alcohol . Cellulose acetate is mainly used in the production of cigarette filters , foils and plastic products. Acetic acid is used as a solvent in the production of terephthalic acid using liquid phase oxidation. It is an important intermediate product in the manufacture of fragrances and medicines .
Other uses include various esters such as acetic acid n butyl acetate and isopropyl acetate , together about 11%, as a solvent for cosmetics and perfumes may be used. Another 10% is used for the production of acetic anhydride, acetanilide , acetic acid chloride and ammonium acetate. Salts such as aluminum diacetate are auxiliaries in the textile and leather industry and are used there for impregnation .
When organochlorosilanes such as dichlorodimethylsilane are reacted with acetic acid, acetoxysilanes are formed. In reaction with silanols , these react with condensation and release of acetic acid to form silicones .
Acetic acid reacts with hydrogen peroxide to form peroxyacetic acid . Industrially, it arises from the oxidation of acetaldehyde with air. Peroxyacetic acid is a strong oxidizing agent that has an antimicrobial effect and is used for disinfection. In addition, peroxyacetic acid epoxidizes various alkenes to epoxides.
| Commercial grades of acetic acid | concentration |
|---|---|
| Glacial acetic acid, acidum glaciale | 99% |
| Vinegar essence | 15-25% |
| Technical acetic acid | 30-50% |
| Crude acetic acid | 40-80% |
| Acetic acid DAB 7 | 99% |
Other uses
The World Health Organization's List of Essential Medicines includes acetic acid as one of the agents used in the treatment of ENT diseases in childhood.
Acetic acid is used in screening for the detection of cervical cancer in sub-Saharan Africa . The acetic acid is applied to the cervix. If the area turns white after about a minute, the test is positive.
Acetic acid is used to acidify hygiene and cosmetic products, such as peeling . The acetic acid causes the top layer of dead skin cells to flake off, leaving a smoother surface. The effect was already used by the Egyptian ruler Cleopatra , whose milk baths also contained acetic acid, which smoothes the skin.
In the photo laboratory practice of “wet” or analogue photography , diluted acetic acid (3–5%) is used to neutralize the developer baths as a so-called “stop bath”. The solution is often mixed with an indicator dye that shows when the stop bath becomes alkaline and therefore ineffective.
Latex, a suspension of natural rubber in an aqueous medium, is coagulated with acetic acid in a low concentration . The charged latex particles repel each other; by adding acetic acid, this charge is neutralized and the latex coagulates.
Glacial acetic acid can be used to prepare calcareous fossils in chalk . The acid is poured over the rock. A reaction cannot take place here because the calcium acetate produced cannot dissolve. Only after dilution does a reaction take place in the entire rock.
Hazard warnings
The classification and labeling according to the dangerous goods regulations depends on the concentration. Glacial acetic acid or solutions with more than 80% acid by mass are assigned to dangerous goods class 8 (corrosive substances) with packaging group II (substances with medium risk). As a secondary risk, dangerous goods class 3 (flammable liquids) must be marked with ( danger label 8/3). Solutions with at least 50% by mass and at most 80% by mass of acid are now only assigned to class 8 (corrosive substances) with packaging group II (substances with medium risk) (label 8). For solutions with more than 10 mass% and less than 50 mass% acid, class 8 (corrosive substances) with packaging group III (substances with low risk) (label: 8) applies.
Pure acetic acid is considered a flammable liquid . Flammable vapor-air mixtures can form above the flash point . The compound has a flash point of 38.5 ° C. The explosion range lies between 6% by volume (148 g / m³) as the lower explosion limit (LEL) and 17% by volume (430 g / m³) as the upper explosion limit (UEL). The maximum explosion pressure is 6.3 bar. The limit oxygen concentration at 200 ° C is 10.6% by volume. The limit gap width was determined to be 1.69 mm. This results in an allocation to gas group IIA. The ignition temperature is 485 ° C. The substance therefore falls into temperature class T1. The odor threshold is 8-10 ppm . At a concentration of around 80%, the corrosive effect corresponds to that of concentrated hydrochloric acid .
According to the Globally Harmonized System for the Classification and Labeling of Chemicals (GHS), acetic acid is classified as a category 3 flammable liquid with a caustic effect on the skin (category 1A). The applicable H and P phrases are H314 (causes severe skin burns and eye damage), H226 (flammable liquid and vapor) and P280 (wear protective gloves / protective clothing / eye protection / face protection), P305 + P351 + P338 (in contact with eyes: Rinse cautiously with water for a few minutes. Remove contact lenses if possible. Continue rinsing) and P310 (call a poison center or doctor immediately).

toxicology
Acetic acid can be absorbed through the digestive tract , breath and skin. Acetic acid is breathed in via the citric acid cycle and the respiratory chain in all cells of the body while generating energy into carbon dioxide ( CO 2 ) and water (H 2 O) as the ultimate metabolic products . Acetic acid can be exhaled through the lungs. Concentrated acetic acid has a strong irritant effect on the skin and mucous membranes. After physical contact with the acid, therefore, appropriate care must be taken to avoid chemical burns, eye damage and irritation of the mucous membranes : skin blisters sometimes only appear hours after exposure. Prolonged skin contact with glacial acetic acid leads to tissue destruction in the affected areas.
Exposure to breathable air for eight hours at a concentration of 10 ppm can cause irritation of the eyes and the nasal and oral mucous membranes as well as irritation of the airways in the throat. Concentrations above 1000 ppm cause severe irritation and cannot be endured for a long period of time. A lethal dose is 20 to 50 grams of acetic acid, in children the value is 5 to 10 grams.
Long-term contact with acetic acid degreases the skin and can lead to eczema . Direct contact of acetic acid with the eyes, e.g. through splashes, can lead to blindness . A sensitization to acetic acid is rare, but occurred.
Acetic acid is easily biodegradable in water and is not bioaccumulative . As an acetate, it is not acutely toxic to fish up to concentrations of 1000 mg / l. It is non-toxic to insects such as the copper colored grave runner up to a discharge rate of 1000 l / ha. In rats, the median lethal dose (LD 50 value) was found to be 3310 mg per kg body weight.
proof
Acetic acid can be detected using the iron chloride test. Acetic acid forms an intense red color with an iron (III) chloride solution.
In 13 C-NMR, measured in deuterochloroform , the carbonyl carbon gives a peak at a chemical shift of 178.12 ppm and the carbon of the methyl group gives a peak at a chemical shift of 20.8 ppm. In 1 H-NMR, measured in deuterochloroform, the hydrogen of the acid function gives a peak at a chemical shift of 11.42 ppm and the hydrogen of the methyl group a peak at 2.098 ppm.
Common and quantitative determination of acetic acid is carried out by means of gas chromatography .
literature
- Hosea Cheung, Robin S. Tanke, G. Paul Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a01_045 .
Web links
- Entry on Acetic acid in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ Entry on E 260: Acetic acid in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on ACETIC ACID in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d e f g h i j k l m n o p q r Entry on acetic acid in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF; 78 kB).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-52.
- ↑ entry to Acetic acid in the Hazardous Substances Data Bank , accessed on May 8 2017th
- ↑ Entry on Acetic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 64-19-7 or acetic acid ), accessed on November 2, 2015.
- ↑ Entry on Acetic acid in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b c Michael Röper: The history of acetic acid production on chemanager-online.com.
- ↑ IUPAC Provisional Recommendations 2004: Chapter P-12.1 (PDF; 346 kB), p. 4.
- ↑ Latin translation of vinegar. on www.pons.com.
- ^ Charles E. Mortimer, Ulrich Müller: Chemistry - The basic knowledge of chemistry. Georg Thieme Verlag, 2003, ISBN 3-13-484308-0 , p. 214.
- ^ A b Carol Ann Rinzler: The Encyclopedia of Cosmetic and Plastic Surgery. Facts on File, 2009, ISBN 978-0-8160-6285-0 , p. 3.
- ↑ Sylvia Feil, Jörg Resag, Kristin Riebe: chemistry in human history. Fascinating chemistry. Springer Verlag, 2017, ISBN 978-3-662-49919-1 , pp. 199–230.
- ^ John Emsley: Nature's Building Blocks: An A – Z Guide to the Elements. Oxford University Press, 2001, ISBN 0-19-850340-7 , p. 229.
- ^ Edvard Hjelt : History of Organic Chemistry: Vieweg, 1916, ISBN 978-3-663-06328-5 , p. 10.
- ^ Meyer's Large Conversational Lexicon . Volume 6, Leipzig 1906, pp. 120-122.
- ↑ Ursula Lang, Sabine Anagnostou: Vinaigre de Quatre Voleurs - legend, secret drug or antiseptic? IGGP Berlin, September 14 - 17, 2011. (PDF)
- ↑ a b c d e Christoph Syldatk : Organic acids. Acetic acid (acetate). In: Garabed Antranikian : Applied Microbiology. Springer, Berlin / Heidelberg, 2006, ISBN 3-540-24083-7 , pp. 344–347.
- ↑ E. Bames, B. Beyer, J. Grossfeld: Handbuch der Lebensmittelchemie. Volume IX: Vinegar, consumer goods and secret agents. Springer, 1942, ISBN 978-3-642-88892-2 (reprint), pp. 4-7.
- ↑ a b c d e Rolf D. Schmid: Pocket Atlas of Biotechnology and Genetic Engineering. 2nd Edition. Wiley-VCH, Weinheim 2006, ISBN 3-527-31310-9 , pp. 18-19.
- ^ Perstorp Holding AB: Annual Report 2005, p. 32.
- ↑ a b H. M. Binburry, W. Elsner: The dry distillation of wood. Verlag Julius Springer, 1925, p. 133.
- ↑ Max Klar: Technology of charring and the manufacture of acetic acid, acetone, methyl alcohol and other wood distillates. Springer, 1903, pp. 116-124.
- ^ Carl Graeb: History of Organic Chemistry. Springer Verlag, 1972, ISBN 3-642-65018-X , p. 148.
- ↑ a b c Hosea Cheung, Robin S. Tanke, G. Paul Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, pp. 4–10, doi : 10.1002 / 14356007.a01_045 (section “Carbonylation of Methanol”).
- ↑ E. Crotti, A. Rizzi, B. Chouaia, I. Ricci, G. Favia, A. Alma, L. Sacchi, K. Bourtzis, M. Mandrioli, A. Cherif, C. Bandi, D. Daffonchio: Acetic Acid Bacteria, Newly Emerging Symbionts of Insects. In: Applied and Environmental Microbiology. Volume 76, 2010, p. 6963, doi: 10.1128 / AEM.01336-10 .
- ↑ Vincent G. Martinson, Brayn N. Danforth, Robert L. Minchley: A simple and distinctive microbiota associated with honey bees and bumble bees. In: Molecular Ecology. Volume 20, 2011, pp. 619-628 doi: 10.1111 / j.1365-294X.2010.04959 .
- ↑ TO Chaudry, PJ Travers, J. Yuenger, L. Colletta, P. Evans, JM Zenilman, A. Tummon: Analysis of Vaginal Acetic Acid in Patients Undergoing Treatment for Bacterial Vaginosis. In: Journal of Clinical Microbiology. Volume 42, 2004, pp. 5170-5175, doi: 10.1128 / JCM.42.11.5170-5175.2004 .
- ^ Benno Kunz: food biotechnology. Behr's Verlag, 2016, ISBN 978-3-95468-276-8 , p. 42.
- ↑ JG Ferry: Enzymology of the fermentation of acetate to methane by Methanosarcina thermophila. In: BioFactors. Volume 6, No. 1, 1997, pp. 25-35. PMID 9233537 (Review).
- ↑ RJ Yokelson et al .: Emissions of formaldehyde, acetic acid, methanol, and other trace gases from biomass fires in North Carolina measured by airborne Fourier transform infrared spectroscopy. In: Journal of Geophysical Research : Atmospheres. Volume 104, 1999, pp. 30109-30125, doi: 10.1029 / 1999JD900817 .
- ↑ Puja Khare, N. Kumar, KM Kumari, SS Srivastava: Atmospheric formic and acetic acids: An overview. In: Reviews of Geophysics . Volume 37, 1999, p. 227, doi: 10.1029 / 1998RG900005 .
- ↑ David M. Mehringer, Lewis E. Snyder, Yanti Miao, Frank J. Lovas: Detection and Confirmation of Interstellar Acetic Acid. In: The Astrophysical Journal . Volume 480, pp. L71-L75, doi: 10.1086 / 310612 .
- ↑ Thomas Bührke: All-Chemie mit dem Radioteleskop ( Memento from August 22, 2017 in the Internet Archive ).
- ↑ Chris J. Bennett, Ralf I. Kaiser: The Formation of Acetic Acid (CH 3 COOH) in Interstellar Ice Analogs. In: The Astrophysical Journal . Volume 660, 2007, p. 1289, doi: 10.1086 / 513267 .
- ^ DE Hibbs, U. Kolitsch, P. Leverett, JL Sharpe, PA Williams: Hoganite and paceite, two new acetate minerals from the Potosi mine, Broken Hill, Australia. In: Mineralogical Magazine. Volume 66, pp. 459-464, doi: 10.1180 / 0026461026630042 .
- ^ The Nobel Prize in Physiology or Medicine 1964.
- ↑ Barbara E. Jones: From waking to sleeping: neuronal and chemical substrates. In: Trends in Pharmacological Sciences. Volume 26, 2005, p. 578, doi: 10.1016 / j.tips.2005.09.009 .
- ↑ D. Elieh-Ali-Komi, MR Hamblin: Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. In: International journal of advanced research. Volume 4, Number 3, March 2016, pp. 411-427. PMID 27819009 , PMC 5094803 (free full text).
- ^ Severian Dumitriu: Polysaccharides in Medicinal Applications . CRC Press, 1996, ISBN 0-8247-9540-7 , pp. 631 ( google.com ).
- ^ Hosea Cheung, Robin S. Tanke, G. Paul Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, p. 1, doi : 10.1002 / 14356007.a01_045 (section “Introduction”).
- ^ Hosea Cheung, Robin S. Tanke, G. Paul Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, pp. 24-25, doi : 10.1002 / 14356007.a01_045 (section “Economic Aspects”).
- ^ FE Fontaine, WH Peterson, E. McCoy, MJ Johnson, GJ Ritter: A New Type of Glucose Fermentation by Clostridium thermoaceticum. In: Journal of bacteriology. Volume 43, No. 6, June 1942, pp. 701-715. PMID 16560531 , PMC 373636 (free full text).
- ↑ Adalbert Wollrab: Organic Chemistry: An Introduction for Teaching and Minor Students. 4th edition. Springer, 2014, ISBN 978-3-642-45143-0 , p. 588.
- ↑ R. Brockhaus: Catalytic gas-phase oxidation of butenes to acetic acid. In: Chemical Engineer Technology - CIT. Volume 38, 1966, p. 1039, doi: 10.1002 / cite.330381005 .
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis. Teubner, Wiesbaden 2007, ISBN 978-3-8351-0088-6 , pp. 283-292.
- ↑ James F. Roth: The production of acetic acid. In: Platinum metals review. Volume 19.1, 1975, pp. 12-14.
- ^ Glenn J Sunley, Derrick J Watson: High productivity methanol carbonylation catalysis using iridium. In: Catalysis Today. Volume 58, 2000, p. 293, doi: 10.1016 / S0920-5861 (00) 00263-7 .
- ↑ Eugen Müller: Newer views of organic chemistry. Springer Verlag, 1957, ISBN 978-3-642-87592-2 , p. 256.
- ^ A b R. E. Jones, DH Templeton: The crystal structure of acetic acid. In: Acta Crystallographica . Volume 11, 1958, pp. 484-487, doi: 10.1107 / S0365110X58001341 .
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry. 5th edition. Georg Thieme Verlag, 2005, ISBN 3-13-541505-8 , pp. 263-264.
- ↑ structural data of R. Boese, D. blowers, R. Latz, A. trees: Acetic acid at 40K. In: Acta Crystallographica . Volume C55, 1999, doi: 10.1107 / S0108270199099862 (CIF file only).
- ↑ Minhua Zhang, Lihang Chen, Huaming Yang, Jing Ma: n Theoretical Study of Acetic Acid Association Based on Hydrogen Bonding Mechanism. In: The Journal of Physical Chemistry A. Volume 121, 2017, pp. 4560-4568, doi: 10.1021 / acs.jpca.7b03324 .
- ↑ R. A. McDonald, S. A. Shrader, D. R. Stull: Vapor Pressures and Freezing Points of 30 Organics. In: J. Chem. Eng. Data . Volume 4, 1959, pp. 311-313, doi: 10.1021 / je60004a009 .
- ^ A b c V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford, 1985, ISBN 0-632-01529-2 .
- ↑ Trade association rules for safety and health at work; BGR 132: Avoidance of ignition hazards due to electrostatic charges. Jedermann-Verlag, Heidelberg 2004, ISBN 3-86825-146-4 .
- ↑ Entry on acetic acid . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed July 20, 2012.
- ↑ a b c d e J. F. Martin, RJL Andon: Thermodynamic properties of organic oxygen compounds. Part LII. Molar heat capacity of ethanoic, propanoic, and butanoic acids. In J. Chem. Thermodynam . Volume 14, 1982, pp. 679-688, doi: 10.1016 / 0021-9614 (82) 90083-0 .
- ^ W. Weltner Jr .: The vibrational spectrum, associative and thermodynamic properties of acetic acid vapor. In: J. Am. Chem. Soc . Volume 77, 1955, pp. 3941-3950, doi: 10.1021 / ja01620a003 .
- ↑ W. V. Steele, R. D. Chirico, A. B. Cowell, S. E. Knipmeyer, A. Nguyen: Thermodynamic properties and ideal-gas enthalpies of formation for 2-aminoisobutyric acid (2-methylalanine), acetic acid, (4-methyl-3-penten-2 -one), 4-methylpent-1-ene, 2,2'-bis (phenylthio) propane, and glycidyl phenyl ether (1,2-epoxy-3-phenoxypropane). In: J. Chem. Eng. Data . Volume 42, 1997, pp. 1052-1066, doi: 10.1021 / je970099y .
- ^ A b J. Chao: Thermodynamic properties of key organic oxygen compounds in the carbon range C1 to C4. Part 2. Ideal gas properties in J. Phys. Chem. Ref. Data 15 (1986) 1369-1436.
- ↑ a b c J. Schmidt: Design of safety valves for multi-purpose systems according to ISO 4126-10. In: Chem. Ing. Techn. Volume 83, 2011, pp. 796-812, doi: 10.1002 / cite.201000202 .
- ↑ Alfons Hädener, Heinz Kaufmann: Fundamentals of general and inorganic chemistry. Birkhäuser Verlag, 2006, ISBN 3-7643-7041-6 , p. 119.
- ↑ Patent US5288318 : Cellulose acetate and starch based biodegradable injection molded plastics compositions and methods of manufacture. Published February 22, 1994 , Inventors: Jean M. Mayer, Glenn R. Elion.
- ↑ Teaching information bakery technology: Microbiological aspects of sourdough fermentation - regulation of acetic acid formation ( as PDF ) .
- ↑ Brigitte M. Gensthaler: Deadly interplay . In: Pharmaceutical newspaper. Volume 30, 2001.
- ↑ Werner Back: Microbiology of food: beverages. Behr's Verlag, 2008, ISBN 978-3-89947-360-5 , p. 237.
- ↑ M.-S. Rhee, S.-Y. Lee, RH Dougherty, D.-H. Kang: Antimicrobial Effects of Mustard Flour and Acetic Acid against Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella enterica Serovar Typhimurium. In: Applied and Environmental Microbiology. Volume 69, 2003, pp. 2959-2962, doi: 10.1128 / AEM.69.5.2959-2963.2003 .
- ↑ Ordinance on the trade in vinegar and vinegar essence, Section 1, Paragraph 1.
- ^ A b Hosea Cheung, Robin S. Tanke, G. Paul Torrence: Acetic Acid. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, pp. 19-20, doi : 10.1002 / 14356007.a01_045 (section “Uses”).
- ↑ Fred Winter: Fragrances and Perfume Technology. Verlag Julius Springer, 1933, p. 94.
- ^ Herman F. Mark: Encyclopedia of Polymer Science and Technology. John Wiley & Sons, 2007, ISBN 978-0-470-04610-4 , p. 1103.
- ^ B. Phillips, PS Starcher, BD Ash: Preparation of Aliphatic Peroxyacids. In: J. Org. Chem. Volume 23, 1958, pp. 1823-1826. doi: 10.1021 / jo01106a001
- ↑ a b Joël Fokom-Domgue, Christophe Combescure, Victoire Fokom-Defo, Pierre Marie Tebeu, Pierre Vassilakos, André Pascal Kengne, Patrick Petignat: Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta- analysis of diagnostic test accuracy studies. In: BMJ. S. h3084, doi: 10.1136 / bmj.h3084 .
- ↑ Rudolf Ditmar: The rubber. Springer, 1912, pp. 25-27.
- ^ Jan Kresten Nielsen, Sten Lennart Jakobsen: Extraction of Calcareous Macrofossils from the Upper Cretaceous White Chalk and Other Sedimentary Carbonates in Denmark and Sweden: The Acid-Hot Water Method and the Waterblasting Technique . (pdf, 762 kB). In: Palaeontologia Electronica. Volume 7, No. 4, pp. 1-11.
- ↑ Osterberg PM, JK Niemeier, CJ Welch, JM Hawkins, JR Martinelli, TE Johnson, TW Root, SS Stahl: Experimental Limiting Oxygen Concentrations for Nine Organic Solvents at Temperatures and Pressures Relevant to Aerobic Oxidations in the Pharmaceutical Industry. In: Org. Process Res. Dev. Volume 19, 2015, pp. 1537-1542, doi: 10.1021 / op500328f .
- ↑ E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ GHS classification criteria acetic acid .
- ↑ a b Karlheinz Lohs, Peter Elstner, Ursula Stephan: Fachlexikon Toxikologie. Springer Verlag, 2009, ISBN 978-3-540-27334-9 , p. 156.
- ↑ NIOSH Pocket Guide to Chemical Hazards: Acetic acid.
- ↑ Registration dossier on Acetic Acid at the European Chemicals Agency (ECHA), accessed on December 26, 2017.
- ↑ Günther Harsch, Rebekka Heimann: The Estercyclus - an experimental project for training resource-conscious thinking and acting. (PDF; 2.6 MB). In: Chem. Sch. Volume 41, 1994, supplement, p. 7.
- ↑ a b Data from the Spectral Database for Organic Compounds .
- ↑ Methods of Organic Chemistry . tape 15 : Synthesis of peptides II. 4th, completely redesigned edition. Georg Thieme Verlag, 1974, ISBN 3-13-216304-X , p. 553 ( google.com ).











![{\ displaystyle \ mathrm {(a) \ [PdCl_ {4}] ^ {2 -} + C_ {2} H_ {4} + H_ {2} O \ rightarrow CH_ {3} CHO + Pd + 2 \ HCl + 2 \ Cl ^ {-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/272108c37754118dfc7ea70418d73f1f4ba6136b)
![{\ displaystyle \ mathrm {(b) \ Pd + 2 \ CuCl_ {2} +2 \ Cl ^ {-} \ rightarrow [PdCl_ {4}] ^ {2 -} + 2 \ CuCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6e5a62f373f2a6e8dc8a4d03e4c022bddbf3a443)