drug
A drug (derived from "Arznei", Middle High German arzenīe , also erzenīe : " Heilkunde , Heilkunst, Heilmittel, Arzneimittel, Pharmazie "; related to " doctor ") or equivalent drug ( Latin medicamentum 'Heilmittel' ), also called therapeutic , is a A substance or a composition of substances that is intended “to cure or prevent human or animal diseases ” or is suitable for influencing physiological functions or enables a medical diagnosis .
This definition is based on the more detailed formulation in two fundamental legal regulations of the European Union, the Directives 2001/83 / EC (Community code for human medicinal products) and 2001/82 / EC (Community code for veterinary medicinal products ). The definition from the EU directives has now been incorporated into a number of national drug laws, including the German drug law .
The term drug from the high-level general language is no longer used in the technical language. The word form Arzenei is out of date. Treatment with drugs is known as medication , drug therapy , drug therapy, or drug therapy / treatment .
Drug term
In the definition of the term medicinal product according to European regulations mentioned in the introduction, the twofold meaning should be emphasized as essential, namely with regard to the
- Purpose of the agent, detached from an actual effectiveness ( drug according to the name , presentation drug )
- Existence of a pharmacological, immunological or metabolic effect in the human or animal organism being treated ( medicinal products according to the effect , functional medicinal products ).
This means that there are both products that are used or should be used for the purpose of healing or disease prevention, detached from an actual effect, to be classified as medicinal products, as well as products that do not claim to be medicinal, but have an effect due to their special composition. This classification is important because drugs come under a different law than food , cosmetics or medical devices (problem of product differentiation ).
Remedies, on the other hand, include other medically supportive measures such as spa treatments , massages , occupational therapy or physiotherapy .
Germany
The German Medicines Act has adopted the European definitions of medicinal products for human and veterinary medicinal products and combined them in Section 2 (1) with only a slight change in the wording .
The so-called “doubtful rule” from Article 2 Paragraph 2 of Directives 2001/83 / EC and 2001/82 / EC has also been adopted by the AMG. It states that the regulations of the Medicines Act are also to be applied if a product falls under the definition of medicinal products as well as the definition of other product groups (e.g. food, dietary supplements , medical products, biocides or cosmetics).
The Medicines Act also defines in § 4 as a special form of a medicinal product the finished medicinal products : These are
[...] Medicinal products that are manufactured in advance and placed on the market in a pack intended for delivery to consumers or other medicinal products intended for delivery to consumers that are otherwise prepared using an industrial process or, except in Pharmacies that are commercially manufactured. Finished medicinal products are not intermediate products that are intended for further processing by a manufacturer.
In contrast to finished drugs, so-called prescription and defective drugs are manufactured in pharmacies .
Furthermore, the AMG differentiates the medicinal products according to their nature or area of application into blood preparations , sera , vaccines , allergens , medicated feed, medicinal products for advanced therapies (also called ATMPs; Advanced Therapy Medicinal Products ), as well as radioactive , xenogenic , homeopathic , anthroposophic and herbal medicinal products.
Austria
In Austria, the European drug definition has not yet been fully implemented. Medicines are defined in Section 1, Paragraph 1 of the Medicines Act:
"Medicines" are substances or preparations made from substances that
- 1. are intended for use in or on the human or animal body and as agents with properties for healing or for alleviating or preventing human or animal diseases or pathological complaints, or
- 2. Applied in or on the human or animal body, or administered to a human or animal, either
- a) to restore, correct or influence the physiological functions through a pharmacological, immunological or metabolic effect, or
- b) to serve as the basis for a medical diagnosis.
The definition is almost identical to that in the German Medicines Act before the 15th Amendment to the AMG came into force in July 2009.
In addition, medicinal products are those objects that contain a medicinal product or to which a medicinal product is applied and which are intended for use on or in the human or animal body, as well as substances and preparations made from substances that do not have the characteristics of paragraph 1, provided they are are intended to be used in the manufacture of medicinal products.
Switzerland
In Switzerland, Article 4 of the Therapeutic Products Act (HMG) defines medicinal products as follows:
Products of chemical or biological origin which are intended or are touted for the medical effect on the human or animal organism, in particular for the detection, prevention or treatment of diseases, injuries and disabilities; Medicines also include blood and blood products .
The Therapeutic Products Act includes, among other things, the term “Medicinal Products for Complementary Medicine”. According to the Complementary and Herbal Medicinal Products Ordinance, this term includes “Asian, homeopathic (including homeopathic- spagyric / spagyric) and anthroposophic medicinal products”; Asian medicines are Chinese , Tibetan or Ayurvedic medicines.
Drug name and classification
In view of the great variety of medicinal products and a vast number of popular names, it is important that these have clear names in order to avoid potentially dangerous confusion. Fixed rules for the designation have therefore been introduced for finished medicinal products. A fancy name proposed by the manufacturer and approved by the authorities, which is also often protected as a trade name , is often used as the name of the drug . Alternatively, the of which is the World Health Organization assigned to the drug -related international non-proprietary name , sometimes used in conjunction with the manufacturer's name or other additives, as a drug name. This is often the case with generic drugs. The non-proprietary name must always be given for medicinal products with up to three active ingredients. The non-proprietary name is not only a freely usable, unambiguous name for the medicinal substance, the class of active substance can also be derived from certain syllables in the name.
Classification systems have been developed for the systematic ordering of the variety of drugs, which classify drugs according to substance groups or diagnosis groups. A widely used system is the Anatomical-Therapeutic-Chemical Classification System coordinated by the World Health Organization . This uses the organs for the main site of action, therapy groups and the chemical structure to categorize substances, i.e. drugs and sometimes combinations. There is an adapted official German version for the German pharmaceutical market.
Drug information
Reliable information on quality, efficacy and safety is essential for the safe use of drugs. In the case of finished medicinal products, the official summary of the product characteristics is a central document from which a large part of the product information is derived. There, for example, the composition, areas of application, dosage, contraindications, side effects and interactions are given. As specialist information , the information is provided by the manufacturers primarily for specialist groups; The content of the package insert for the consumer is also derived from this document. Regulations on the designation of drugs and on mandatory information that the manufacturer must provide for users and consumers are regulated in drug law.
Pharmaceutical Directory
Drug directories make it easy to find information about drugs. Commercial directories such as the Red List or the Yellow List, which specify the composition of finished medicinal products, their field of application, the dosage, the manufacturer, the price and other details, are widely used. Official, freely accessible drug information systems have been set up to inform the public, including EudraPharm for the EU and PharmNet.Bund for Germany. In Austria, the Austria Codex and the register of medicinal specialties are authoritative. For Switzerland there is the Swiss Medicines Compendium . Other internationally important lists are the British National Formulary and the World Health Organization's list of essential medicinal products .
Pharmacopoeia
The pharmacopoeia covers a different area of information than the drug registers. It records the common medicinal substances (synonym: active pharmaceutical ingredients), auxiliary substances and dosage forms . There, all the specifications (qualitative and quantitative limit values) and test methods that are important for drug testing are described in detailed monographs. For systematic reasons, finished medicinal products are generally not recorded in the pharmacopoeia.
development
Medicines are usually not pure substances, but preparations of medicinal substances with auxiliary substances and are now for the most part developed and manufactured by pharmaceutical companies as so-called finished medicinal products .
The development of new drugs includes the identification and non-clinical development of new active ingredients (drugs), the galenic development of drug forms and clinical testing . Because of the extensive and lengthy required efficacy and compatibility tests, the development of an innovative drug is very expensive. New active ingredients are therefore patented by the developers , so they can only be used commercially by the patent holder. Only after the patent protection has expired can other companies bring cheaper generic preparations with the same active ingredient onto the market. Only a few innovative new drugs come onto the market each year. The development of a drug without a new active ingredient, for example a new drug form of a known drug or a generic, is far less expensive.
Non-clinical development
In the non-clinical development, new active ingredients are identified, improved and examined for their suitability as medicinal substances in suitable experimental pharmacological test systems. The development includes an extensive toxicity determination including mandatory animal tests .
The new substances can be synthetic substances or natural substances from plants, animals or microorganisms. Large libraries of molecules are often searched, from which in the end only a few new substances are suitable for further development. Since the end of the 20th century, genetically engineered substances have also been used more and more .
The pharmaceutical technology or Galenic developed the dosage form of a finished drug from one (or more) drugs and one (or more) auxiliary materials . The galenics can influence the rate of release, the location of the release and the location of the effect.
Clinical trial
For the approval of a human medicinal product, its testing in human clinical trials is required. These include:
- Phase I: Review of the uptake of the medicinal substance and first qualitative reviews of the (side) effects on a small group i. d. Usually healthy subjects (approx. 10–50 subjects).
- Phase II: Qualitative and quantitative reviews of the effects and side effects of a drug and dose determination for Phase III of the clinical trial (approx. 100–500 patients).
- Phase III: Quantitative proof of the effectiveness of a drug compared to a placebo or another control under defined conditions (usually over 1,000 patients).
- (Phase IV): After approval has been granted, drugs are further investigated in long-term studies.
Most experimental drugs fail in the course of clinical trials. Only about 8% of the funds examined in phase I ultimately achieve approval.
Manufacturing
The production of drugs includes the production of the drug in its desired dosage form, but also the procurement of the raw materials and packaging as well as quality assurance. Today, most of the production takes place industrially in pharmaceutical companies ; the in-house production of drugs in pharmacies (and, as in the Middle Ages, by surgeons and lay doctors) only plays a minor role.
Dosage forms
Medicinal substances are usually not used as a pure substance, but rather prepared in a special pharmaceutical form . The preparation should allow safe dosing when used and optimize the effect of the drug. For this purpose, the medicinal products are processed with auxiliary substances in various manufacturing processes. The most commonly manufactured forms are tablets and capsules ; together they make up about 80% of the drugs used. In addition to solid drug forms, liquid forms for injection or infusion , as well as for oral use (e.g. syrup), ointments for external use, and aerosols and solutions for inhalation are also used ; other special forms are only rarely used.
production
The manufacturing processes of pharmaceutical technology are very different depending on the dosage form. In the most common form, the tablet, for example, the starting materials are weighed in and mixed, usually granulated for better processing and only then pressed dry into tablets. These are then often provided with a coating that gives them special properties such as lubricity, acid resistance, shape and color.
Production must take place under controlled, germ-free conditions. Medicines for injection must be made sterile . The production of biological medicinal products is particularly complex , as small deviations in the process can lead to large differences in the potency. The entire production process is monitored by ongoing quality assurance. The quality of the drug, in particular its identity, content and purity, must be checked. All manufacturing steps must be documented in detail.
Packaging and labeling
The packaging, including its labeling, is an integral part of a finished medicinal product . It must be approved as part of the approval process. Solid dosage forms are often dispensed in transparent packaging (blisters) that are packed in folding boxes.
In the European Union , finished medicinal products must be labeled with the name of the medicinal product, the strength and the dosage form, the international non-proprietary name, the name of the pharmaceutical company, the package size , the expiry date and the batch number . Instructions for storage are also prescribed (e.g. out of the reach of children, possibly certain storage temperatures). In addition, other labels may be required by law or commercially required from country to country.
In Germany, for example, the pharmaceutical central number with code 39 , which is important for logistics and billing, is used. For this purpose, the relevant central associations of pharmacies and health insurance companies have contractually agreed in accordance with Section 300 of Book V of the Social Code (Fifth Book) to use the PZN as an identification key for billing pharmaceuticals. For this purpose, the pharmacies transfer the PZN of the drugs dispensed at the expense of the GKV in machine-readable form to the prescription sheets. The pharmaceutical trade uses PZN for ordering, invoicing, logistics and warehousing. PZN are used as article / order numbers in (electronic) purchase orders between industry, pharmaceutical wholesalers and pharmacies, on delivery notes and invoices. They support route planning and tracking, the identification of the delivered goods at goods receipt and in the warehouse. In this context, the subject of bar coding plays a central role.
Legislation
The manufacture of pharmaceuticals is regulated by national, European and international regulations. This is to ensure that the population is supplied with high quality pharmaceuticals. Pharmaceutical manufacturers require an official manufacturing license, for the issue of which suitable and sufficient rooms, technical facilities and control options must be available. In the European Union, a qualified person must certify for the manufacturer that each batch of a medicinal product has been manufactured and tested in accordance with the specifications and regulations. Compliance with good manufacturing practice , which prescribes proper, hygienic, well-documented and controlled manufacturing, is checked by official inspections.
traffic
According to the German AMG ( Section 4 , Paragraph 17), the so-called placing on the market includes "keeping in stock for sale or other distribution, offering for sale, offering for sale and distribution to others." In many countries, the trade in drugs is governed by drug law strictly regulated. Medicines may only be placed on the market after they have been approved by the authorities. The dispensing of pharmaceuticals to end consumers is regulated differently for different categories of pharmaceuticals. The advertising of medicinal products subject to a separate law in many countries.
Admission
In many countries, finished medicinal products require official approval before they can be placed on the market. The purpose of this regulation is to protect the population from harmful or ineffective drugs. The pharmaceutical company must prove the effectiveness, safety and quality of the drug in an application for approval. For approval by the competent drug authority, it is crucial that the assessment of the documents for the drug results in a favorable risk-benefit ratio . The documentation to be submitted to the authority with the application is regulated in detail.
Uniform procedures have been introduced in the European Union that allow approval in several member states. For certain drugs, including genetically engineered drugs and drugs with new active ingredients against HIV, cancer, diabetes and some other diseases, applications to the European Medicines Agency are mandatory for EU-wide approval. For other medicinal products, the application for approval can be submitted to the relevant national authority, in Germany to the Federal Institute for Drugs and Medical Devices or the Paul Ehrlich Institute , in Austria to the medical market supervision based in the Austrian Agency for Health and Food Safety (AGES).
Switzerland carries out authorizations independently of the EU; Swissmedic is responsible for this . In the USA , approval is granted by the FDA .
In recent years, specific regulations and procedures for the approval of orphan and pediatric medicines have been put in place in the EU and US to encourage development in these neglected areas.
The prescription of an approved drug in an indication or patient group outside the granted approval is called off-label use . The prematurely tolerated use of a drug that has not yet been approved for humanitarian reasons is also called compassionate use .
Submission
The distribution of pharmaceuticals to the end consumer is strictly regulated. Pharmaceutical companies and pharmaceutical wholesalers usually only supply pharmacies and hospital pharmacies ; the vast majority of medicinal products may only be dispensed to end consumers by pharmacies. This is to ensure that consumers receive expert advice.
In Germany, drugs can be divided into four groups according to the dispensing authorization:
- over the counter, d. H. Non-pharmacy-only (also sold outside pharmacies)
- pharmacy- only (available only in pharmacies)
- Prescription only (only available in pharmacies on presentation of a medical, dental or veterinary prescription )
- Narcotics (dispensing in pharmacies only upon presentation of a narcotic prescription )
The first two groups of drugs are collectively referred to as OTC drugs (from English "over the counter" = over the counter). In 2007, of all drugs approved in Germany, 60% were prescription-only, 34% pharmacy-only and 5% over-the-counter. Narcotics made up one percent.
There are five tax categories in Switzerland :
- Category A: single dispensing on medical prescription; Category A + are narcotics with a special prescription
- Category B: Dispensed on medical prescription
- Category C: dispensing in pharmacies
- Category D: dispensing in pharmacies and drugstores
- Category E: Submission without specialist advice
In 2005, 59% of all pharmaceuticals approved in Switzerland fell into categories A and B, 10% into category C and 31% into categories D and E.
The requirements that must be met in order to be able to dispense medicinal products vary from country to country. In Germany, only pharmacists are allowed to own pharmacies and dispense pharmacy- only medicinal products for human use. In some other European countries, including Austria and some cantons of Switzerland, certain doctors also have this right of dispensation . Veterinarians are also allowed to dispense veterinary drugs from a veterinary pharmacy in Germany .
application
Medicines are used under a wide variety of circumstances, from self -medication to administration by specialists under intensive inpatient supervision. In any case, the choice of an agent suitable for the field of application is decisive. Medicines can be given in different dosage forms for different types of application.
field of use
From a medical point of view, it is crucial to use a drug that is suitable for the area of application, the so-called indication . In doing so, contraindications or contraindications and drug interactions must be observed. Prescribing a drug outside of the areas of application specified in the approval ( off-label use ) requires the patient to be given special information by the doctor.
The drug groups most frequently prescribed in Germany in 2007 were ACE inhibitors and sartans for the treatment of high blood pressure , antibiotics for bacterial infections, anti-inflammatory and anti-inflammatory drugs , pain relievers , beta-blockers for cardiovascular diseases and antidiabetics for diabetes mellitus .
regulation
The vast majority of medicinal products are used on medical prescription. When issuing a prescription, doctors are advised to take into account not only the medical necessity, but also the possibility of non-drug treatment, economic efficiency and other legal requirements. If doctors fail to carry out the required complete medical control when using drugs that can be life-threatening, this can lead to professional sanctions. The drug prescription should be preceded by an individual risk-benefit assessment for the patient.
Application type
There are a number of different types or forms of application for medicinal products . For example, one can differentiate between internal and external, local and body-wide applications. The most common is oral administration through the mouth as it is easy to perform by the patient independently and appropriate dosage forms are inexpensive to manufacture. Injection medication is more time-consuming and usually has to be carried out by medical professionals. For certain chronic diseases such as diabetes mellitus, patients can be trained to give themselves injections of insulin preparations, for example .
quality control
Quality assurance measures are necessary to avoid damage from improper use of drugs . In addition to the respective specialist information, doctors can use manufacturer-independent therapy guidelines for prescribing them , which present the state of the art for many diseases. IT solutions can provide appropriate information in the event of inconsistencies between diagnoses and prescriptions or in the event of expected interactions. When dispensing drugs, pharmacists are obliged to check the medical prescription for errors and to check the quality of the drug to be dispensed. Nursing staff should apply principles such as the so-called 5-R rule when administering and administering drugs: right patient, right drug, right dosage and / or concentration, right type of application, right time.
Many patients need to take several medicines in the course of a day. Medication dispensers are often used to make it easier to take . Another possibility is the individual blistering of drugs by a pharmacy. Here, the various prescribed finished medicinal products for the patient are repackaged in transparent packaging. According to the German Medicines Act, no approval is required for such blistering ( Section 21 , Paragraph 2, 1b. (B))
The drugs prescribed for a patient and the corresponding intake instructions are documented in a medication plan.
Effects
Medicines and the medicinal substances they contain interact with the human or animal body in a variety of ways. Pharmacology studies these interactions . It studies the time course of the amount of drug in the body after administration as well as the strength and the mechanisms of action at the site of action and ultimately the effects on the body as a whole. The desired effects of drugs (“effectiveness”) are often accompanied by side effects, which are mostly undesirable. The weighing of the benefit against the risk must always be weighed up individually.
Pharmacokinetics
Pharmacokinetics is the time course of the concentration of a drug in the body after the drug has been administered. In order to be able to exert their effect in the body, drugs or the drugs they contain must be given the opportunity to reach the site of action. For example, tablets must first disintegrate in the stomach or intestines after being swallowed, then the released drug must be absorbed and absorbed into the blood. Distribution in the body occurs through the bloodstream , which also transports the drug to the site of action. At the same time, other bodily functions have an effect on the drug. It can be changed or broken down by the metabolism. This biotransformation can make the drug ineffective, but it can also produce harmful degradation products. Finally, drugs or their breakdown products are also excreted again, usually via the kidneys or bile .
Pharmacodynamics
Pharmacodynamics examines the effect of the drug at the site of action. By definition, drugs have a pharmacological, immunological or metabolic effect on body functions. This is usually done through highly selective interactions with certain parts of the body. The mechanism of action of most drugs is based on binding to specific target structures in the body (there are several hundred different target structures in the human body). These are often membrane receptors or enzymes whose function is activated or inhibited by the drug. This influences the body's function and the course of the disease.
The strength of the pharmacological effect usually depends on the dose of the drug. This can be represented in a dose-response relationship . Not all effects of a drug are wanted. The so-called therapeutic range of a drug can be derived from the dose-response relationships for the desired and undesired effects . This is a measure of safety: the greater the therapeutic range, the safer the drug is to use.
effectiveness
The effectiveness of a drug is the sum of its desired effects for the respective area of application. Medicines are used in particular to detect, prevent, alleviate or cure diseases. This goal goes far beyond a purely pharmacological effect. Depending on the intended use, medicinal products should have a therapeutic efficacy, a diagnostic suitability or a preventive effect that has been proven in clinical studies . This is a prerequisite for drug approval.
Not all drugs can cure diseases or treat the causes of the disease. Often the effectiveness is limited to an improvement of symptoms .
Side effects
The effectiveness is almost always accompanied by side effects . A side effect (also known as "undesirable drug effect" in technical terms) is an effect that occurs during normal use and is harmful and unintentional. Known side effects are documented in the package insert along with their frequency.
The balancing of benefits and risks is a key problem in the development, approval and use of drugs. In severe cases, side effects can lead to permanent physical harm. If such damage occurs after the medicinal product has been used as intended, the pharmaceutical company can be held liable.
Interactions
The way drugs work is complicated by the fact that different drugs can affect each other. These drug interactions (interactions) can lead to the complete loss of effectiveness of a drug or to serious side effects. A distinction is made between the following types of interaction:
- pharmacodynamic interaction - interaction of drugs at the same site of action (or affecting the same body function)
- pharmacokinetic interaction - interaction in absorption, distribution, processing by metabolism or excretion
- pharmaceutical interaction - interaction based on intolerance to substances
- Some foods can also affect the way certain medicines work.
Drug interactions are a major problem, especially in multimedia , since the likelihood of interactions increases rapidly with the number of drugs. In this context, in addition to the general creation of greater clarity, a detailed medication plan , which according to the new e-health draft law is to be issued by doctors from October 2016, should be considered useful. In this way, potential interactions can be researched more easily and experienced doctors can possibly recognize at a glance what can otherwise be lost due to the situation and excitement-related medication forgotten in the doctor's consultation, to the detriment of therapy safety and success.
abuse
Because of their effects, some drugs are also repeatedly misused. The drug abuse often associated with dependence, for example benzodiazepines and opiates, is particularly problematic . In Germany, the number of drug addicts is estimated at 1.4 to 1.9 million.
Another area in which drugs are misused is doping . The use of illegally obtained questionable drugs and misuse lead to serious drug damage time and again.
monitoring
After approval, finished medicinal products are continuously monitored for the occurrence of undesirable effects in order to record unexpected, previously unknown and rare side effects. The reason for this is that only a few thousand patients were treated at the time of approval, which means that rare side effects cannot be recorded. Even long-term damage is usually not foreseeable at the time of approval.
Doctors and pharmacists must therefore report suspected cases of such side effects. Pharmaceutical entrepreneurs are legally obliged to report adverse effects - even if they are only suspected cases - to the approval authority either at short notice or at prescribed reporting times, depending on their severity. You must regularly analyze and evaluate databases and the literature and report the results to the responsible authorities, which in turn assess the reports. In Germany, the BfArM or the PEI then take appropriate measures according to a step-by-step plan process to avert health risks. These can be requirements for drug information or restrictions on approval; in rare cases, drugs are even withdrawn from the market entirely.
Economics and Health Systems
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Medicines are among the most important tools in medicine. On the one hand, they are an important economic asset that in some industrialized countries makes a significant contribution to the balance of trade, and on the other, they are a major cost factor for health systems. For this reason, in many countries a dense network of specific regulations for pricing and the assumption of costs has emerged. These are also not harmonized in the EU.
Pharmaceutical expenditure in all OECD countries was over US $ 650 billion in 2007. This means that an average of 15% of all health expenditure was accounted for in this area, which is the third largest cost factor after hospital treatment and outpatient care. At the same time, pharmaceutical spending has risen by almost 50% internationally, also adjusted for inflation, and is therefore an important cost driver.
In 2007, the US spent the most per capita on pharmaceuticals by far, followed by Canada and Greece. Germany and Switzerland are in the middle, slightly above the OECD average.
The world's most expensive drug, which was developed by Novartis for the treatment of spinal muscular atrophy , received approval in the USA in May 2019.
Germany
In 2004, drugs to the value of € 36.1 billion were dispensed. Two thirds of the costs were borne by the statutory health insurances, a further 22% by private households. One third of the household expenses were deductibles , the rest was self-medication . In 2004, the costs of pharmaceuticals at the expense of the statutory health insurance were moderate in an international comparison at € 308 per insured person. A generic share of over 50% of the prescriptions contributes to this.
In 2012, the statutory health insurance funds in Germany spent 29.4 billion euros on pharmaceuticals; in 2018 this was 38.7 billion euros.
| rank | Trade name | Active ingredient | million Euro |
|---|---|---|---|
| 1 | Humira | Adalimumab | 844 |
| 2 | Xarelto | Rivaroxaban | 691 |
| 3 | Eliquis | Apixaban | 633 |
| 4th | Revlimid | Lenalidomide | 569 |
| 5 | Zytiga | Abiraterone acetate | 340 |
In the case of prescription drugs, the sales prices are regulated by the drug price regulation. The high price level of pharmaceuticals is criticized.
According to SGB V, insured persons in the German statutory health insurance are essentially entitled to the supply of prescription drugs. This requirement is specified in the G-BA's Drugs Directive . In order to curb the growth in pharmaceutical expenditure, fixed amounts have been introduced for many pharmaceutical groups that determine the maximum price that is covered by the statutory health insurance. In 2010 the law for the reorganization of the pharmaceutical market (AMNOG) was passed: health insurers can negotiate discounts with manufacturers via pharmaceutical discount contracts . Also was cost-benefit assessment of drugs by the IQWiG introduced the G-BA may limit the supply of individual drugs on the basis thereof. In 2011 and 2012 a total of 120 million euros were saved.
| rank | Trade name | Active ingredient | Million packs |
|---|---|---|---|
| 1 | Novaminsulfone | Metamizole | 24.2 |
| 2 | Ibuflam | Ibuprofen | 19.1 |
| 3 | RamiLich | Ramipril | 12.2 |
| 4th | L-thyroxine Henning | Levothyroxine | 9.7 |
| 5 | Amlodipine dexcel | Amlodipine | 7.8 |
According to the Federal Institute for Drugs and Medical Devices (BfArM), delivery bottlenecks in the drug supply have increased significantly since the 2010s. While only 40 new drugs with delivery problems were reported to the BfArM as the supervisory authority in 2013, there were already 264 drugs in 2018. Thomas Preis, head of the North Rhine Pharmacists' Association, stated that in 2019 pharmacies found on average significantly more than 100 drugs that were not available. The reason is that fewer and fewer manufacturers / factory locations are producing an active ingredient and more and more production has been relocated abroad for economic reasons. For 2018 there were 133 delivery bottlenecks at a single university clinic in Germany, Frank Dörje, President of the Federal Association of German Hospital Pharmacists, illustrated the magnitude of the problem. Seriously ill patients are particularly affected. After all, the Drug Supply Strengthening Act (VSG) 2017 obliged manufacturers to report their own delivery bottlenecks to hospitals immediately. In March 2019, the BfArM last published a “List of active substances classified as relevant to supply or with an acutely increased supply risk”.
Switzerland
In 2012, pharmaceuticals to the value of CHF 5.649 billion were dispensed, not including the drugs dispensed in inpatient hospitals, the cost of which is estimated at CHF 700 million. Medicines make up 22% of the outpatient costs of compulsory health insurance . The per capita expenditure in 2012 was 700 francs. Basic insurance only pays drugs in the list of specialties, a positive list for drugs that also contains maximum prices up to which costs are covered. In 2016, spending on pharmaceuticals at the expense of basic insurance rose by around 6 percent compared to the previous year. They amounted to over CHF 7 billion, which corresponds to around a quarter of the costs of basic insurance. In Switzerland, 54% of sales are made with patent-protected original drugs, around 20% with originals without patent protection and around 20% with generics. Deductibles that favor generics over original products were introduced in 2006 and are intended to encourage their use.
In Switzerland, drug prices are regulated by the Nursing Services Ordinance. Price comparisons between European countries show that pharmaceuticals are particularly expensive in Switzerland.
Austria
When it comes to pharmaceuticals, Austria is considered a “low-cost country”. In 2007, drugs worth € 2.822 billion were dispensed at the expense of the statutory health insurance. Medicines (with VAT) thus accounted for 21.4% of the expenditure by health insurance carriers. In 2008, pharmaceutical expenditure per inhabitant was € 326 excluding VAT. Medicines must be listed in the reimbursement code if they are to be paid for by social security; inclusion in the code is often linked to price agreements. In most cases, patients participate in the drug costs through a prescription fee .
Drug residues
Due to their intended use, drugs usually contain highly biologically active substances that are often relatively stable and only slowly break down in the body or in the environment. Since unintentional ingestion of drugs can lead to health problems, their residues must also be taken into account.
Residues in food
Veterinary medicinal products used on food- producing animals can pose a risk to humans. That is why the use of drugs in animal production is regulated by law. When veterinary medicinal products are approved, health risks from residues are assessed and food safety is assessed. In the EU, for example, Regulation (EC) No. 470/2009 on maximum residue levels of pharmacologically active substances in food of animal origin defines maximum residue levels that are considered to be harmless to humans. After using veterinary medicinal products, prescribed waiting times must often be observed before the treated animal can be used in food production. Food is continuously checked for drug residues by the authorities on a random basis.
Residues in the environment
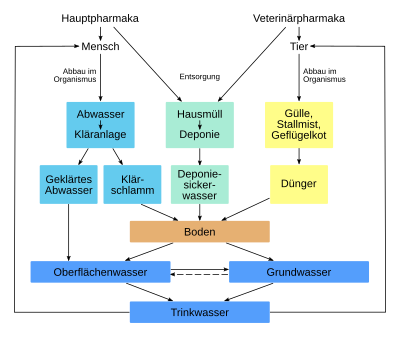
Medicines, their ingredients and their breakdown products enter the environment in a variety of ways, where they can endanger humans, animals and ecosystems.
In addition to excretions (urine, feces) from humans and animals, sources of input include the improper disposal of unused drugs via the sewer system, for example via the toilet, sink or rinsing containers with drug residues. Contrary to the widespread assumption, the disposal of small amounts of old pharmaceuticals via household waste is harmless to the environment from the perspective of waste management. Since 2005, municipal waste has been incinerated in Germany or the pollutants it contains have been inactivated by pre-treatment. Only then is it deposited. The safety aspect alone - household rubbish bins are often freely accessible - makes this disposal method seem questionable. The Federal Environment Agency therefore advises that expired or unused medication be returned to pharmacies or pollutant collection points. Pollutant vehicles can also be used. If the instruction leaflet contains disposal instructions, these must be followed.
Since around the mid-1990s, improved analysis techniques have increasingly been used to detect drug residues in surface , ground and drinking water . However, the concentrations are so low that an acute pharmacological effect is not to be expected. On the other hand, the quantities are in waters well with those of pesticides comparable. A possible ecotoxicity is therefore increasingly considered. With the application for approval of a drug, the applicant has therefore also had to submit an environmental risk assessment for several years. At the level of the European Union , the protection of waters against pharmaceutical residues has been considered in the Water Framework Directive since 2013 together with other trace substances .
In 2019, between 35 and 69 active pharmaceutical ingredients and metabolites were detected in all 40 rivers in Austria . Antibiotic residues in the environment are viewed particularly critically , as it is feared that bacteria will develop antibiotic resistances that limit the effectiveness of antibiotics against pathogens.
There are various approaches to reducing the drug load in wastewater. Sewage treatment plants are to be improved and sewage sludge no longer applied to the soil. Another approach is to develop sustainable active ingredients that are biodegradable in the environment. Further possibilities lie in the prevention of infections, a reflected prescribing practice or also in the integration of the topic of drugs in the environment in medical training professions.
According to the Federal Environment Agency, 30,000 tonnes of human medicinal products are consumed annually in Germany, with 2,300 active substances . Of these, 1,100 active ingredients are ecologically harmless, such as vitamins , electrolytes and peptides, and there are 1,200 active ingredients and an annual consumption of 8,100 tons that are classified as "environmentally relevant". 88% of the residues in surface water originate from excretions, 10% from improper disposal of pharmaceuticals and 2% from the manufacturing process. Up to 47% of patients would at least occasionally improperly dispose of their leftover drugs. One of the most frequently used drugs, the anti-inflammatory drug diclofenac , has an annual consumption of 14 million daily doses and 90 tonnes of annual sales in Germany, of which 60–70% enter the wastewater through natural excretion and so far cannot be clarified or broken down there. The sartanes as blood pressure medicines can be found to a large extent in the waste water again, up to 70 tonnes per year, and are considered "human toxicological risk." Other drugs with high sales and higher concentrations in sewage are ibuprofen (also an anti-inflammatory), carbamazepine (used against convulsions ), ethinylestradiol (a hormone preparation ) and sulfamethoxazole (an antibiotic ).
Veterinary drugs
There are some legal peculiarities for veterinary medicinal products, which are largely based on the fact that many animals are used to produce food . For reasons of consumer protection , pharmaceuticals must be used with particular care in food-producing animals; certain pharmaceuticals are not even allowed. The existing national legal provisions in the EU countries, which implement the requirements of Directive 2001/82 / EC (Community Code for Veterinary Medicinal Products), will be replaced in January 2022 by the directly applicable Regulation (EU) 2019/6 on veterinary medicinal products . Central aspects of veterinary medicinal products, which are regulated EU-wide by “clear, detailed and directly applicable provisions” of the new regulation, relate in particular to veterinary prescribing, prescription management and monitoring systems for antibiotics , mail-order sales and reallocation .
history
Medicines have been used for thousands of years. Herbal medicinal drugs and some other substances were already in use in ancient times. For a number of medicinal plants there are references to their use from prehistoric times. Already in a grave of a Neanderthal man ( Shanidar IV., In today's Iraq), which was created about 70,000–40,000 years ago, there are additions that, according to pollen studies , can be assigned to seven medicinal plants, which is why the grave of a healer, a shaman with attributes of his activity is suspected. If this find is still isolated from the earliest times , a number of finds are known from the Neolithic , the younger Stone Age, which suggest the use of medicinal plants. In addition to herbal medicinal products, there are also animal and mineral ones.
Antiquity
The medicinal form of the pill, called Cataputon, was mentioned in the Corpus Hippocraticum as early as the time of the advanced cultures of Mesopotamia and Egypt . The Roman encyclopedia Aulus Cornelius Celsus took over the Greek word as catapotium and gave the size of the pill that of a pea or bean. The current name “pill” goes back to Gaius Plinius Secundus and is derived from Pilula, the diminutive of Pila. The pills were then made by hand kneading the drug.
The second popular dosage form were leak juices, which were already mentioned in Babylonian times and were called "eclegma" in Greece. They were made by grinding the medicine and mixing it with honey. They were administered to the patient in a shell. Pliny described that one must let eclegma melt on the tongue. The Greek doctor Pedanios Dioskurides prescribed leak juices as lung therapeutics. Eclegma can by Galen in the groups Diamoron ( mulberry juice ), Diacodyon, Diacaryon (juice of nuts) and Succus de scillae ( sea onion juice ) are divided. Diamoron and Diacaryon were used by Galen and Paulos of Aegina as wound and throat ointments . Related to the leaking juices were the electuaria (Greek: to lick up), which mainly contain antidotes for poisoning such as B. Snakebites were used. In Babylonian medicine they were also used for lung and breast diseases. Nikandros von Kolophon (2nd century BC) reports in his works " Theriaca " and "Alexipharmaca" on the composition and effects of the antidotes. Examples of electuaries are the remedy for snakebites "(Antidotum) Mithridat (i) um" by Mithridates Eupator of Pontus , as well as the further developed form "Theriaca Andromachi" by Andromachus, Nero's personal physician . In addition, electuaria were also used as laxatives, which emerges from the works of Alexander von Tralleis , Marcellus Empiricus and Theodorus Priscianus .
In Egyptian medicine in the “ Ebers Papyrus ”, powder formulations for internal and external use as well as tooth powder are handed down. Powders are also described in the Corpus Hippocraticum . Pedanios Dioskurides describes numerous formulations for external use in skin diseases, as well as eye and nose powders. According to Dioscurides , powders are used internally for stomach and abdominal diseases.
Ointments have already been mentioned in the Ebers Papyrus and are used both in medicine and in cosmetics. Pliny reports that the Persians in particular were keen ointment users. According to the Corpus Hippocraticum, they were composed of honey, water, milk, wine, oils or fats, mixed with drugs and then applied to the skin. In Roman tradition, ointments appear exclusively as cosmetics that were mixed by not highly respected ointment manufacturers (unguentuarii).
In ancient times, plasters (emplastrum) were also used for abscesses, boils and external diseases. The Diachylon plaster , which the Greek personal physician of Emperor Tiberius Menekrates from Zeofleta (4 AD), was known. invented and is also described by Galen . Paulos of Aigina divided the plasters into adhesive plasters, scarring, dividing, softening, ripening plasters, pull plasters and soothing plasters. The production of paving was considered very complicated at the time. A simpler variant were pasty envelopes known as cataplasms , in which the medicinal substances were mixed with medicinal substance carriers .
A drug form of Egyptian medicine that is no longer known today is incense, carried out on individual organs such as the nose, vagina and anus. Resins, frankincense, myrrh, benzoin and other substances were burned and the smoke passed into the organ. The incense "Kuefi" consisting of myrrh, juniper berries, frankincense, Cyprus mastic branches, fenugreek, calamus and styrax was particularly popular. Incense and special incense cones are also described in the Corpus Hippocraticum . Celsus and Dioscurides also report on it. Paulos of Aegina narrated that smoking opens the brain and clears blockages there.
The following authors are particularly important for the remedies of Western medicine:
- Theophrastus of Eresos (371–287 BC) described 550 plants, including numerous medicinal and poisonous plants .
- Pliny the Elder (23 / 24–79 AD) wrote a very extensive encyclopedic natural history , the Naturalis historia . The remedies occupy a large space, almost 1000 are described from the plant kingdom.
- The drug theory De materia medica of the Roman military doctor Dioscurides (1st century), written in five books, is the most extensive of antiquity. It deals with medicinal products from all three natural kingdoms; 102 mineral, 101 animal and 813 herbal medicinal products are described. The work was published around 78 AD and was effective for centuries. In the Middle Ages in particular , it served as a model and treasure trove for other relevant compendia.
Medicines in Greco-Roman medicine were in the context of a therapy to support the natural healing process. On the one hand, “simple” medicinal products ( Simplicia ) were used, some of which were based on folk medicine and some on empiricism. On the other hand, new compounds were created from the mixture of Simplicia , which became more and more popular in Roman times, as well as the use of exotic ingredients. Exotic ingredients were expensive due to their wide origins and were often considered to be particularly effective because of their high value. Scribonius Largus ' compositions were also created in this historical context .
middle Ages
The mediaeval sources for the medicinal treasure are numerous. These include the didactic poem Liber de Cultura Hortorum or Hortulus by Walahfrid Strabo (9th century), who was the abbot of the Reichenau monastery . The knowledge about the healing powers of plants is conveyed in the form of poems ( hexameters ). Another didactic poem about medicinal plants and influenced by the Hortulus is the Macer floridus . The author, Odo von Meung , lived in the 11th century . A Thuringian-Silesian prose translation and editing, the “Older German Macer”, passed down from the 13th century onwards, was widespread and, along with other sources, served as the textual basis for the “ Gart der Gesundheit ” of 1485, one of the most influential printed herbal books . In addition, from around the year 1000 onwards, the European Middle Ages became known with ancient writings that were believed to be lost or forgotten through translations from Arabic into Latin. The centers of translation work are in southern Italy (Salerno) and Spain (Toledo). In addition, there are independent findings from Arab scholars. Abu Bakr Mohammad Ibn Zakariya al-Razi (865 to around 930), Avicenna (980-1037) and other Arab authors are among the most respected authorities in European medicine. Her writings describe heretofore unknown medicinal drugs , for example ambergris , benzoin , cubeb , galangal , camphor , musk , nutmeg , mummy (see Mumia ), sandalwood , senna leaves and others.
Irrespective of the ancient or Arab influence, new, independent observations are made here and there that enrich knowledge about the medicinal treasure. Outstanding are the Physica by Hildegard von Bingen and a font by Albertus Magnus with the title De vegetabilibus .
Early modern age
Since the early modern period , the European medicinal treasure has expanded considerably. After the discovery of the sea route to the East Indies by Vasco da Gama and the landing in America by Columbus , a new dimension opened up in the trade in medicinal plants and drugs. For example, brook root , cinchona bark , curare , guaiac and balsam of Peru came to Europe.
The alchemy of the Arabs was also influential , as a medical goal came to the fore here: the search for the panacea , universal medicine . The most important pioneer for the use of (al) chemical preparations in medicine was Philippus Theophrastus Bombastus von Hohenheim, called Paracelsus (1493–1541). He was the first to advocate the internal use of chemicals , especially poisonous antimony and mercury preparations . Although his teachings found only a limited number of followers during his lifetime, his successors, the Paracelsists , conveyed his ideas to an ever-growing circle of doctors and other scholars. Paracelsus developed remedies based on alcohol, which - later improved by Valerius Cordus - were very popular as Hoffmann drops (ether and alcohol) since 1560.
Johann Rudolph Glauber (1604–1670) presented sodium sulphate and tried it out as a remedy. Sodium sulphate is also called "Glauber's salt" after him. He found the antiseptic properties of phenol and isolated alkaloids with sulfuric acid and nitric acid. This made him an early representative of pharmaceutical chemistry .
19th and 20th centuries


At the beginning of the 19th century, the medicinal treasure was initially reduced again: What remained were medicines, the effectiveness of which was considered certain according to the current state of science. Their manufacture was reserved exclusively for pharmacists.
The increase in knowledge in chemistry led to the fact that an abundance of effective ingredients was isolated from medicinal plants , such as the alkaloids quinine , morphine and strychnine . Not only alkaloids , but also many other phytonutrients were isolated and a large number of them were used medicinally. This was made possible by the introduction of new apparatus and devices in the pharmacy: in 1823 the pharmacist Petit from Corbeil invented the pulverizing drum and in 1833 the Parisian pharmacist Pierre Francois Guillaume Boullay (1777–1869) invented the percolation method, which displaced the extraction press.
Towards the end of the 19th century, the triumphant advance of the organic-synthetic pharmaceuticals developed by the tar color industry, whereby the manufacturing process was subject to patent protection . This promoted the industrial production of medicinal specialties, the pharmaceuticals manufactured in ready-to-sell packaging, as they dominate the picture today and largely replace the individual manufacture in the pharmacy. The acetylsalicylic acid , known as aspirin, many other pain killers and others on the nervous system acting drugs belong here ( anesthetics , antiepileptics , Parkinson drugs , psychotropic drugs and others). Other examples are drugs that affect the autonomic nervous system , such as sympatholytics (which include beta blockers ), which are used to treat cardiovascular disease . The number of synthesized active ingredients quickly became unmanageable.
As a result of biochemical , physiological and clinical-chemical investigations of the 19th and 20th centuries, there have been numerous advances in hormones and vitamins . Among other things, the basics for the therapeutic use of vitamins, insulin , sex hormones ( estrogens , gestagens , the “ pill ”, androgens ), hormones of the adrenal cortex ( glucocorticoids such as cortisone), thyroid hormones , tissue hormones and their antagonists (for example antihistamines) were established as antiallergic drugs ).
Medicines for the prevention and therapy of infectious diseases , based on the concept of the magic ball by Paul Ehrlich from 1900, became particularly important. These include above all antibiotics , vaccines as well as disinfection and sterilization agents . With their help, but also through better nutrition and housing as well as improved hygiene , once life-threatening diseases ("scourges of humanity"), which can be traced back to microorganisms, have decreased significantly. The research by Paul Ehrlich (1854–1915) ( arsphenamine ) and Gerhard Domagk (1895–1964) ( sulfonamides ) should be mentioned here. In addition, it was discovered that natural substances such as penicillin, which is formed by mold , can be used successfully as antibiotics against these diseases.
See also
literature
Pharmacology and toxicology
- Ernst Mutschler , G. Geisslinger, Heyo K. Kroemer , S. Menzel, P. Ruth: drug effects: pharmacology - clinical pharmacology - toxicology. 10th edition. Scientific publishing company, Stuttgart 2012.
history
- Nicholas Eschenbruch, Viola Balz, Ulrike Klöppel, Marion Hulverscheidt: Medicines of the 20th Century. Historical sketches from cod liver oil to thalidomide. Transcript, Bielefeld 2009, ISBN 978-3-8376-1125-0 .
- Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history. 2., revised. and exp. Edition. Stuttgart 2005, ISBN 3-8047-2113-3 .
- Wolf-Dieter Müller-Jahncke , Christoph Friedrich : History of drug therapy. Deutscher Apothekerverlag, Stuttgart 1996, ISBN 3-7692-2038-2 .
- Rudolf Schmitz: History of Pharmacy. Volume I: From the beginning to the end of the Middle Ages. Govi-Verlag, Eschborn 1998, ISBN 3-7741-0706-8 .
- Rudolf Schmitz, Wolf-Dieter Müller-Jahncke , Christoph Friedrich : History of the pharmaceutical industry. Volume II: From the early modern era to the present. Govi-Verlag, Eschborn 2005, ISBN 3-7741-1027-1 .
- Wolfgang Schneider: Lexicon for the history of medicines: Specialized dictionary for the history of pharmaceutical botany, chemistry, mineralogy, pharmacology, zoology . 7 volumes (volume 5 in three parts), Govi Verlag, Frankfurt am Main 1968 to 1975 (volume 1: animal drugs, volume 2: pharmacological drug groups, volume 3: pharmaceutical chemicals and minerals, volume 4: secret drugs and specialties, volume 5: Herbal Drugs, Volume 6: Supplements to Volume 3, Volume 7: Register).
Critical literature on medicines
- Marcia Angell: The Pharma Bluff. How innovative the pharmaceutical industry really is. Kompart, Bonn / Bad Homburg 2005, ISBN 3-9806621-9-5 .
- Markus Grill: Sick Businesses - How the Pharmaceutical Industry Manipulates Us. Rowohlt, Reinbek 2007, ISBN 978-3-498-02509-0 .
- Sonia Shah: Tested on humans! Redline Wirtschaft, Munich 2008, ISBN 978-3-636-01561-7 .
- Ben Goldacre: The Pharmaceutical Lie: How Pharmaceutical Companies Mislead Doctors and Harm Patients. (Title of the American original edition 2012: Bad Pharma ) Kiepenheuer & Witsch, 2013, ISBN 978-3-462-04577-2 .
Drug metabolism
- Karl-Heinz Beyer: Biotransformation of Medicines. Springer Verlag, Berlin / Heidelberg / New York / London / Paris / Tokyo / Hong Kong 1990, ISBN 3-540-50696-9 .
- G. Gordon Gibson, Paul Skett: Introduction to Drug Metabolism. Chapman & Hall, London / Glasgow / New York / Tokyo / Melbourne / Madras 1986, ISBN 0-412-26390-4 .
- S Preissner: database Cytochrome-Drug interactions (Drug metabolism). Nucleic Acids Research , 2010, accessed August 20, 2011 .
Web links
- Old medicines - a waste for the bin . Website of the Federal Environment Agency for the disposal of pharmaceuticals
Individual evidence
- ↑ Early New High German Dictionary . Vol. 2 (1994), col. 206-210.
-
↑ Complete definition:
"a) All substances or compositions of substances that are intended as agents with properties for the cure or prevention of human or animal diseases, or b) all substances or compositions of substances that are used in or on the human or animal body or a people and animals can be administered to either the human or animal physiological functions by exerting a pharmacological , immunological or metabolic restore effect of correcting or modifying or to making a medical diagnosis. "
Summary wording of the definition in the Community code on human medicinal products, Directive 2001/83 / EC in the consolidated version of November 16, 2012 and the definition in the Community code for veterinary medicinal products, Directive 2001/82 / EC in the consolidated version of August 7, 2009 , which differ only in relation to humans and animals ( see respectively Article 1). - ↑ Directive 2001/83 / EC in the consolidated version of November 16, 2012 .
- ↑ Directive 2001/82 / EC in the consolidated version of August 7, 2009 .
- ↑ Remedy . Duden online
- ↑ Arzenei on zeno.org
- ↑ medication . Duden online
- ↑ Presentation drug website of the Federal Institute for Drugs and Medical Devices (BfArM), glossary, accessed on May 3, 2018
- ↑ Ordinance of the Swiss Agency for Therapeutic Products on the Simplified Authorization of Complementary and Herbal Medicinal Products (Complementary and Herbal Medicinal Products Ordinance) HTML .
- ↑ Johannes Arends: Popular names of drugs, medicinal herbs, medicines and chemicals. 17th edition. Berlin / Heidelberg 2001.
- ↑ Directive 2001/83 / EC Article 1 (20).
- ↑ a b Directive 2001/83 / EC Article 54.
- ^ Franz Bracher, Frank Dombeck: What international non-proprietary names say. In: Pharmaceutical newspaper. 45 (2002) (online)
- ↑ Scientific Institute of the AOK : Anatomical-therapeutic-chemical classification (ATC) .
- ↑ Directive 2001/83 / EC Article 11.
- ↑ Österreichischer Apotheker-Verlag. Retrieved August 25, 2018 .
- ↑ Register of medicinal specialties. Federal Office for Safety in Health Care , accessed on August 25, 2018 .
- ↑ a b c Achim Aigner, Frank Czubayko, Gerhard Klebe, Milton Stubbs: The eye of the needle - from research to development. In: Dagmar Fischer, Jörg Breitenbach (Ed.): The pharmaceutical industry. 3. Edition. Spectrum Akademischer Verlag, Heidelberg 2010, ISBN 978-3-8274-2129-6 .
- ↑ Wolfgang Wegner: 'Freiberg Medicines Teaching'. In: Werner E. Gerabek u. a. (Ed.): Encyclopedia of medical history. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 437.
- ^ A b Robert Becker: The medicinal substance a chance - the dosage form. In: Dagmar Fischer, Jörg Breitenbach (Ed.): The pharmaceutical industry. 3. Edition. Spectrum Akademischer Verlag, Heidelberg 2010, ISBN 978-3-8274-2129-6 .
- ↑ a b Tobias Jung: People, Processes, Material - Production. In: Dagmar Fischer, Jörg Breitenbach (Ed.): The pharmaceutical industry. 3. Edition. Spectrum Akademischer Verlag, Heidelberg 2010, ISBN 978-3-8274-2129-6 .
- ↑ Directive 2001/83 / EC in the consolidated version of January 26, 2007 , accessed on December 4, 2011 , Article 40.
- ^ A b Manfred Schlemminger: The proof of the pudding - the admission. In: Dagmar Fischer, Jörg Breitenbach (Ed.): The pharmaceutical industry. 3. Edition. Spectrum Akademischer Verlag, Heidelberg 2010, ISBN 978-3-8274-2129-6 .
- ^ A b c d Erwin Deutsch , Andreas Spickhoff: Medical Law. Chapter XXVIII: Pharmaceutical Trade. 6th edition. Springer, Berlin 2008, ISBN 978-3-540-72467-4 .
- ↑ Information about over-the-counter medicines according to Section 50 of the Medicines Act. Retrieved July 17, 2018 .
- ^ A b c d Michael Simon: The health system in Germany. An introduction to structure and functionality. Chapter 7: The Drug Supply. 2nd Edition. Huber, Bern 2008, ISBN 978-3-456-84135-9 .
- ^ Fridolin Marty: Medicines In: Gerhard Kocher, Willy Oggier (Ed.): Health Care Switzerland 2007–2009. Huber, Bern 2007, ISBN 978-3-456-84422-0 .
- ^ Ulrich Schwabe, Dieter Paffrath (Ed.): Drug Ordinance Report 2008 Chapter 1: Drug Ordinance 2007 at a Glance. Springer, Heidelberg 2008, ISBN 978-3-540-69218-8 .
- ^ Medicines commission of the German medical profession : Medicinal prescriptions. Chapter A: Rules and advice for the regulation. 20th edition. Deutscher Ärzteverlag, Cologne 2003, ISBN 3-7691-1140-0 .
- ↑ VG Berlin, judgment of November 2, 2011 , Az. 90 A 3.08, full text.
- ^ Rainer Lasek, Bruno Müller-Oerlinghausen : Therapy recommendations of the drug commission of the German medical profession. In: Z Arztl Advanced Training Qualitatssich. Volume 91, 1997, pp. 375-383; PMID 9340209 .
- ↑ Antje Jelinek, Rainer Danz (Ed.): Medicines. Textbook for nursing professions. Chapter 3: Handling Medicines. Fischer & Urban, Munich 2005, ISBN 3-437-26290-4 .
- ↑ a b c d Ernst Mutschler, Gerd Geisslinger, Heyo Kroemer, Peter Ruth, Monika Schäfer-Korting: drug effects. 9th edition. Knowledge Verlagsges., Stuttgart 2008, ISBN 978-3-8047-1952-1 .
- ↑ Directive 2001/83 / EC in the consolidated version of January 26, 2007 , accessed on December 4, 2011 , Article 1 (2).
- ^ Karl Feiden, Hermann Pabel: Dictionary of Pharmacy Volume 3: Drugs and Pharmacy Law. Knowledge Verlagsges., Stuttgart 1985, ISBN 3-8047-0670-3 .
- ↑ Directive 2001/83 / EC in the consolidated version of January 26, 2007 , accessed on December 4, 2011 , Article 1 (11).
- ↑ Drug Commissioner of the Federal Government: Drug and Addiction Report 2009 (PDF)
- ^ Medicines Commission of the German Medical Association: Pharmacovigilance. In: Medicinal prescription in practice. Volume 32, special issue April 2005. akdae.de (PDF)
- ^ World Health Organization: The World Medicines Situation. (1,052KB PDF) September 8, 2004, accessed December 31, 2012 (English).
- ↑ a b OECD: Health at a Glance 2009 Chapter 7.4 .: Pharmaceutical Expenditure. OECD, Paris 2009, ISBN 978-92-64-07555-9 .
- ↑ USA approve Novartis drugs - the most expensive drug in the world. In: srf.ch . April 25, 2019. Retrieved May 26, 2019 .
- ^ A b Peter Thielen: Financially not yet a great success . In: Handelsblatt . No. 102 , May 31, 2013, p. 14 .
- ^ Health care data: Medicines. October 21, 2019, accessed November 23, 2019 .
- ↑ a b MEDICINAL PRODUCTS IN THE CHANGE - FROM SMALL MOLECULES TO COMPLEX BIOLOGICS. arznei-telegram , November 15, 2019, accessed on November 23, 2019 .
- ↑ More delivery bottlenecks: drugs often not available , ARD Tagesschau from July 28, 2019, accessed July 28, 2019
- ↑ Supply bottlenecks for pharmaceuticals are increasing, Ärzteblatt from June 12, 2019, accessed July 28, 2019
- ↑ Delivery bottlenecks: List of supply-relevant active ingredients (excluding vaccines) , BfArM as of March 28, 2019, accessed July 28, 2019
- ↑ a b c d Andreas Schiesser: Medicines In: Willy Oggier (Ed.): Health Care Switzerland 2015–2017. 5th edition. Hogrefe, Bern 2015, ISBN 978-3-456-85441-0 (E-Book (PDF) ISBN 978-3-456-95441-7 ) pp. 243-266.
- ↑ Helsana: Helsana Pharmaceutical Report 2017 creates transparency in the pharmaceutical sector In: helsana.ch, November 20, 2017.
- ↑ a b c Austrian Chamber of Pharmacists: The Austrian Pharmacy in Figures 2009 (PDF).
- ↑ Federal Office for Consumer Protection and Food Safety on residues of veterinary drugs in food, accessed on December 4, 2011.
- ↑ Dispose of old drugs responsibly. In: Waste Manager Medicine. Retrieved August 7, 2018 .
- ↑ Federal Office for the Environment : Medicines in Groundwater In: bafu. admin.ch , accessed on September 16, 2018.
- ^ Pharmaceuticals in the Environment — Global Occurrences and Perspectives. doi : 10.1002 / etc.3339
- ↑ Bavarian State Office for the Environment: Medicines in the Environment. (PDF) 2008 (PDF).
- ↑ Manfred Clara, Christina Hartmann, Karin German: Active pharmaceutical ingredients and hormones in rivers . GZÜV special measurement program 2017/2018. ( bmnt.gv.at [PDF; accessed on November 2, 2019]).
- ^ Radka Alexy, Klaus Kümmerer: Antibiotics in the environment. In: KA - sewage, waste . Volume 52 (5), 2005, pp. 563-571.
- ↑ Sewage Sludge Ordinance (AbfKlärV): Ordinance on the recycling of sewage sludge, sewage sludge mixture and sewage sludge compost. In: Waste Manager Medicine. Retrieved August 7, 2018 .
- ^ Medicines and chemicals in water: Interview with Prof. Kümmerer, Leuphana University Lüneburg. In: Waste Manager Medicine. Retrieved August 7, 2018 .
- ↑ Vera Zylka-Menhorn: Medicinal residues in water: Avoidance and elimination Deutsches Ärzteblatt 2018, Volume 115, Issue 22 of June 1, 2018, pages A1054-A1056
- ↑ M. Jung: New EU regulation for veterinary medicinal products , Pharmazeutische Zeitung, January 7, 2019.
- ↑ Wolfgang Schneider : Lexicon for the history of medicines: specialized dictionary for the history of pharmaceutical botany, chemistry, mineralogy, pharmacology, zoology . 7 volumes (volume 5 in three parts), Govi Verlag, Frankfurt am Main 1968 to 1975 (volume 1: animal drugs, volume 2: pharmacological drug groups, volume 3: pharmaceutical chemicals and minerals, volume 4: secret drugs and specialties, volume 5: Herbal Drugs, Volume 6: Supplements to Volume 3, Volume 7: Register).
- ↑ Cf. Johann Heinrich Dierbach : Die Arzneimittel des Hippokrates, or attempt at a systematic enumeration of the medicaments occurring in all Hippocratic writings. Karl Groos' new academic bookshop, Heidelberg 1824.
- ^ A b Wolf-Dieter Müller-Jahncke, Christoph Friedrich, Ulrich Meyer: Medicinal history . 2., revised. and exp. Edition. Knowledge Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 19 .
- ^ Wolf-Dieter Müller-Jahncke, Christoph Friedrich, Ulrich Meyer: Medicinal history . 2., revised. and exp. Edition. Knowledge Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 20 .
- ^ Wolf-Dieter Müller-Jahncke, Christoph Friedrich, Ulrich Meyer: Medicinal history . 2., revised. and exp. Edition. Knowledge Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 21 .
- ^ Wolf-Dieter Müller-Jahncke, Christoph Friedrich, Ulrich Meyer: Medicinal history . 2., revised. and exp. Edition. Knowledge Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 22 .
- ^ Christian Schulze: Therapy . In: Karl-Heinz Leven (Hrsg.): Ancient medicine, a lexicon . Beck, Munich 2005, ISBN 3-406-52891-0 , Sp. 855-859, here col. 856 f .
- ↑ Peter Dilg: Medicinal Treasure . In: Karl-Heinz Leven (Hrsg.): Ancient medicine, a lexicon . Beck, Munich 2005, ISBN 3-406-52891-0 , Sp. 98/99 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 495.