antibiotic
An antibiotic (formerly also antibiotic , from Greek ἀντί- anti- "against" and βίος bios "life"; plural : antibiotics , antibiotics ) in the original sense is a naturally formed, low-molecular-weight metabolic product of fungi or bacteria , which even in low concentrations Inhibits the growth of other microorganisms or kills them. An antibiotic in the broader sense is also an antimicrobial substance that does not occur in nature and is partly obtained synthetically , fully synthetically or genetically , but not as a disinfectant .
Antibiotics and their derivatives are widely used as anti-infectives ( drugs used to treat infectious diseases ).
In common parlance, the term antibiotics mostly refers to medicinal substances or drugs used to treat bacterial infectious diseases . Together with agents against infectious diseases caused by protozoa ( antiprotozoic agents ), against fungi ( antimycotics ), against viruses ( antivirals ) and worms ( anthelmintics ), they form the group of therapeutic agents against infectious diseases (anti-infectives).
To the subject
The term antibiotic is derived from antibiosis , a term introduced by Paul Vuillemin in 1889 for a relationship between living beings that has disadvantages for one of the parties involved by inhibiting its growth and / or reproduction or by killing it.
Although antibiosis has been known for a long time, the synthetic arsphenamine is considered to be the first therapeutically antimicrobial substance . The term chemotherapeutic for chemical-synthetic antimicrobial substances comes from the time of the large-scale synthetic production of sulfonamides . It was only later that penicillin was introduced as the first naturally occurring antimicrobial agent in antibacterial therapy. Today, both substances of biological origin (antibiotics in the original sense) and synthetically produced chemotherapeutic agents are generally referred to as antibiotics.
In addition to the chemical definition, the definition is now also broader in the biological sense. It does not limit the biogenic origin of antibiotics only to microorganisms such as fungi and bacteria, but also includes substances such as phytoalexins and defensins from more highly organized organisms such as plants and animals; The presence of endogenous antibiotic substances is also known in humans.
history
background
The discovery and application of antibiotics are among the most important developments in medical history and have also been described in novels. Louis Pasteur formulated the phrase "life prevents life" after he recognized in 1877 that some types of bacteria prevent each other from growing.
Soon's eye ointment
In 2015, an English research team examined the so-called Bald's Leechbook from the 10th century. They found out that an eye ointment made according to a recipe mentioned therein had a bactericidal effect in vitro against the bacterium Staphylococcus aureus . In a model made from infected mouse tissue, she was able to significantly reduce the germ count of a multi-resistant hospital germ Staphylococcus aureus ( MRSA ). The ointment contained a preparation of garlic, onions, wine and ox bile .
1893–1897: preliminary work by Gosio and Duchesne
Bartolomeo Gosio isolated mycophenolic acid in 1893 from a mold of the genus Penicillium , which he was even able to produce in crystalline form. Gosio observed that he could use it to hinder the growth of the anthrax pathogen. He published this work in 1893 and again in 1896; however, they were not noticed internationally, probably because he wrote in Italian.
Also thirty years before Alexander Fleming , the "official" discoverer of penicillin , the French military doctor Ernest Duchesne wrote his doctoral thesis on his medical student observation that certain molds have antibiotic - that is, bacteria- killing - properties. Today he is considered to be the first to discover the antimicrobial effectiveness of molds. His research was stimulated by the observation that the Arab grooms employed in the military hospital kept the saddles for the horses in a dark, damp room in order to encourage the formation of mold. When Duchesne asked why they were doing this, the stable boys replied that it would heal more quickly the wounds caused by chafing the saddle. In 1896 Duchesne prepared a solution from these mold cultures and injected it into several sick guinea pigs . It was found that all test animals recovered after the injection was given.
Duchesne then studied the interaction between Escherichia coli and Penicillium glaucum in a series of meticulously conducted experiments. It turned out that in a culture that contained only these two species, the fungus was able to eliminate the bacterium. Furthermore, it was found that an experimental animal that had been inoculated with a typhus bacillus in a normally fatal dose showed no signs of disease and was therefore completely healthy - provided it had also previously been inoculated with Penicillium glaucum (in this regard, the results differ von Duchesne depends on the results of Fleming: The strain Penicillium notatum discovered by Fleming showed no effects on typhoid).
His doctoral thesis entitled Contribution à l'étude de la concurrence vitale chez les micro-organismes: antagonisme entre les moisissures et les microbes ("Investigations into the struggle for survival of microorganisms: the antagonism of molds and microbes"), which he wrote in 1897 Was the first scientific work that dealt with the possibilities of a therapeutic use of molds due to their antimicrobial properties. At that time, the Pasteur Institute rejected the doctoral thesis of the then completely unknown and just 23-year-old. Duchesne urged more research, but military service prevented him from developing further activities in the field. It was not until 1949, five years after Alexander Fleming had received the Nobel Prize , that Duchesne was honored posthumously for his services by the French Académie nationale de médecine .
1910: Paul Ehrlich and the arsphenamine
In many cases , arsphenamine, introduced by Paul Ehrlich in 1910, is still regarded as the first antibiotic in history to be discovered. Its spectrum of action was limited to spirochetes ( narrow-spectrum antibiotic ); for the first time, it enabled an effective and relatively safe therapy for syphilis, which was widespread at the time .
Unlike the later penicillin, arsphenamine and its successors, which were manufactured in Germany until 1945 (including sulfamidochrysoidin , initially introduced as an anti-infective agent in 1934 , trade name Prontosil ) were not based on molds, but on artificially produced dyes. Arsphenamine has now been replaced by newer active ingredients in modern medicine. The next antibiotic that was brought onto the market in 1935 was the sulfonamide discovered by Gerhard Domagk .
1928: Alexander Fleming discovered penicillin
The rediscovery of penicillins by Alexander Fleming began in 1928 with a forgotten and moldy staphylococcus culture at St. Mary's Hospital in London, when he discovered that a mold ( Penicillium notatum ) grew on the breeding ground of the bacterial culture , causing the bacteria to multiply in the vicinity of the Had prevented fungus. He called the substance that kills bacteria from the nutrient medium penicillin and published his findings in 1929 in the British Journal of Experimental Pathology .
Since Penicillium notatum (today Penicillium chrysogenum ) as the next medically used antibiotic could not be chemically synthesized, in contrast to the antibiotics mentioned above, but had to be produced by microorganisms (fungi), the treatment of the first patient with penicillin did not take place until 1941 after it was obtained in chemically sufficiently pure form by the Oxford group around Ernst B. Chain and Howard W. Florey .
Since 1941: widespread dissemination and research until today
The real triumph of antibiotics in medicine began with penicillin. The success of penicillin led to the search and discovery of many other antibiotics: streptomycin , chloramphenicol , aureomycin , tetracycline and many others. Most of the antibiotics known today are derived from natural substances .
The best-known "producer" of antibiotics is the mold Penicillium chrysogenum (formerly P. notatum ). His product, penicillin, is now a synonym for antibiotics in the lay language. Even today, the numerous medicinally used antibiotics are biotechnologically produced by bacteria such as the streptomycetes . Another very large group of antibiotics are semisynthesis products that have been chemically modified, but are also derived from natural producers. It is not uncommon for such substances to be produced fully synthetically using modern chemical methods. H. a biotechnological process step is completely dispensed with.
In the 1970s and 1980s, research in the field of antibiotics increased. Today antibiotics are among the most frequently prescribed drugs worldwide , with a market share of thirteen percent, they form the largest single area after all of our drug consumption has been recorded. Of the approximately 8,000 antibiotic substances known today, only about 80 are used therapeutically. According to the BfArM, a total of 2,775 antibiotic preparations were approved in Germany in 2005 . In 1987, 10 to 15 of these preparations had a market share of around four fifths of total sales. In 1997 the proportion of penicillin was 9%.
Effect of antibacterial antibiotics
There are basically two types of effect:
- bacteriostatic (bacteria are prevented from multiplying, but not killed)
- bactericidal (bacteria are killed, for example by bacteriolysis , i.e. the dissolution of their cell wall)
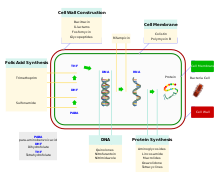
The starting point for the desired effect are structures or mechanisms of the bacterial cells that do not occur in animal or human cells. For example, the effect can take place by inhibiting bacterial cell wall synthesis, protein synthesis on the ribosome , DNA replication or folic acid synthesis . Bacteria are the only known organisms whose cell wall is made of murein . This sugar is only found in bacteria - no other known living being can produce murein. Furthermore, bacteria have different ribosomes for protein biosynthesis and different enzymes for DNA replication than humans. Human cells do not produce folic acid like bacteria do, but take it in with food . Only in this way is it possible that antibiotics are comparatively well tolerated by humans.
The variety of bacteria on which an antibiotic acts, its spectrum of action, varies depending on the antibiotic. Antibiotics, which have a very broad spectrum of activity, i.e. which act on a large number of different bacteria, are called broad spectrum antibiotics .
According to the chemical structure, a distinction is made between different groups of antibiotics:
β-lactams
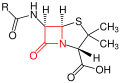
β-lactam antibiotics (β-lactams for short) bind firmly ( covalently ) and irreversibly to certain penicillin binding proteins (PBP), which are responsible for the formation of peptide bonds in the bacterial cell wall component murein . Various such PBPs, such as the D-alanine transpeptidases , are known as targets for β-lactam antibiotics. Blocking these enzymes disrupts murein biosynthesis so that the important cell wall components cannot be produced again. The resulting lesions (“holes”) create instability in the cell wall and the high osmotic pressure leads to lysis and thus to cell death. Under extreme conditions (laboratory) one can actually observe 'bursting' bacterial cells.
Basic structure of penicillins - the β-lactam ring is marked in red .
Basic structure of the cephalosporins - the β-lactam ring is marked in red .
In principle, β-lactam antibiotics have a bactericidal effect . Typical representatives are penicillins , cephalosporins , monobactams and carbapenems . For a finer subdivision, see the article β-lactam antibiotics .
β-lactam antibiotics are combined with β-lactamase inhibitors for certain applications . β-lactamase inhibitors block the β-lactamases produced by some bacteria, which would render β-lactam antibiotics ineffective by cleaving them. Fixed combinations are clavulanic acid + amoxicillin , sulbactam + ampicillin , tazobactam + piperacillin .
Glycopeptides
Glycopeptides also belong to the bacteriolytic antibiotics. They only act on gram-positive bacteria. Similar to β-lactams, they inhibit the biosynthesis of the bacterial cell wall component murein ( peptidoglycan ), but the mechanism of action is different. By complexing terminal D-alanyl- D-alanine sequences of the peptidoglycans, glycopeptides prevent their elongation and cross-linking, in that the transglycosylase is inhibited. The growing bacterial cell wall creates perforations (holes) through which, due to the high osmotic pressure difference, uncontrolled water flows into the bacterial cell ( diffuses ), which is why it eventually bursts. Medicinal substances from the group of glycopeptides are, for example, vancomycin , dalbavancin and teicoplanin .
After administration of an initial standard dose, the maintenance dose is determined on the basis of the serum level in the blood, especially in the case of simultaneous therapy with aminoglycosides, in patients with impaired or inadequate kidney function and in the case of high doses and / or longer treatment periods.
Polyketides
- Tetracyclines
Tetracyclines work against gram-positive and gram-negative bacteria. Tetracyclines inhibit bacterial protein synthesis by attaching themselves to the 30 S-ribosome subunit and thereby preventing the attachment of tRNA . The mode of action is therefore bacteriostatic. The affinity of the tetracyclines for bacterial ribosomes is much greater than that for mammalian ribosomes. Tetracyclines form complex compounds with polyvalent cations such as calcium ions , such as those found in milk and milk products in particular, or magnesium ions (e.g. contained in over-the-counter antacids ) and are less readily absorbed.
- Glycylcyclines
Glycylcyclines act in the same way as the structurally related tetracyclines, but with a significantly higher ribosomal affinity. This has a particular effect on protection proteins.
- Macrolide antibiotics
Macrolide antibiotics bind to the 50 S-ribosome subunits . They block the tunnel through which newly formed polypeptide chains leave the ribosome. As a result, protein synthesis can only take place in a few cycles (around four) and then comes to a standstill, which is why macrolide antibiotics are also called translation inhibitors. These antibiotics have a bacteriostatic effect. An example of this is erythromycin .
- Ketolides
Similar to macrolide antibiotics, ketolides use the 50S ribosome subunits, but they also interact with the 23S rRNA, which inhibits translational activity. They also work by inhibiting the formation of the 30S subunit.
Lincosamides
Lincosamides bind to the 50-S subunit of the ribosomes , where they inhibit protein synthesis in bacteria. Due to the similar mode of action as macrolides , cross-resistance can occur. Lincosamides have a bacteriostatic effect.
Aminoglycoside antibiotics
Aminoglycosides also interfere with bacterial protein synthesis. They attach to the 30 S-ribosomes while protein synthesis is still taking place. Proteins are produced that the bacterium cannot use and that even hinder the construction of the cell wall. These antibiotics have a bactericidal effect.
Polypeptide antibiotics
Polypeptide antibiotics work in the cell membrane. The transport mechanisms are disturbed here, which is why substances harmful to cell function are no longer filtered out. The polypeptide antibiotics include the polymyxins , bacitracin, and tyrothricin .
Lipopeptide antibiotics
Different subgroups of lipopeptides show antibiotic properties. Depending on the subgroup, the antibiotic effect occurs through pore formation in the pathogen, through blockade of cell wall synthesis (in fungi) or through inhibition of protein biosynthesis.
Epoxy antibiotics
Epoxide antibiotics are irreversible inhibitors of the enzyme MurA (UDP- N -acetylglucosamine enolpyruvyl transferase). MurA catalyzes the first step of murein biosynthesis : the transfer of an enolpyruvyl unit from phosphoenolpyruvate (PEP) to UDP- N -acetylglucosamine (UNAG). The effect is bactericidal.
Quinolone antibiotics
Quinolone antibiotics (short: quinolones ) are exclusively produced synthetically. In terms of their principle of action, they belong to the gyrase inhibitors . The enzyme DNA gyrase is indispensable in the bacterium for the untwisting of the DNA strands and leads to a reduction in internal molecular tensions during DNA replication. The administration of gyrase inhibitors inactivates this enzyme, so that the DNA can no longer be untwisted and consequently cannot be replicated. Some quinolones ( ciprofloxacin , norfloxacin ) are also known to be poorly absorbed by the body and have a weaker effect in the presence of polyvalent cations (e.g. Ca 2+ from milk or dairy products or Mg 2+ from antacids).
Streptograms
Streptograms exist in two biochemically different subgroups. Both bind to the P-site of the 50S subunit of the ribosome and thus inhibit the elongation of protein synthesis. Subgroups A and B considered individually are only bacteriostatically effective. Used together (typically in a 70:30 mixture), a bactericidal synergism is shown which , due to a conformational change of the 50S subunit after binding of A-streptogramins, multiplies the activity of B-streptogramins. Group A streptograms alone block an early phase of elongation by blocking the donor and acceptor sites on the ribosome. It is only possible for them to bind to the ribosome if it is free from a bound acyl-tRNA . Group B streptograms, however, can occupy the ribosome in any phase of protein synthesis and thus lead to an inhibition of elongation and to the release of incomplete peptides .
Sulfonamides
Sulfonamides are also known as growth factor analogs. They disrupt nucleic acid synthesis by interfering with the folic acid cycle . The mode of action is bacteriostatic.
Oxazolidinones
Oxazolidinones inhibit the beginning of the synthesis of a peptide strand at the point where the ribosome , messenger or messenger RNA and start tRNA- AS complex assemble. They mostly have a bacteriostatic effect on Gram-positive bacteria; As a representative active ingredient, cycloserine also inhibits Gram-negative bacteria and Mycobacterium tuberculosis .
Ansamycine
Ansamycins (the most important subgroup are the rifamycins ) work by irreversibly binding to the beta subunit of the prokaryotic DNA-dependent RNA polymerase. In this way they block the binding of the enzyme to the DNA and thus the initiation of chain formation. By suppressing RNA transcription, protein synthesis in bacteria is ultimately inhibited. The effects of ansamycins are bactericidal.
Nitroimidazoles
Nitroimidazoles act via reactive intermediates - in - especially in low-oxygen, and only to a small extent in cells with normal oxygen supply reduction of the nitro group formed. These have a bactericidal effect by causing DNA strand breaks in susceptible pathogens (obligatory anaerobic bacteria , certain protozoa ) . The reactive reduction products of the newer bicyclic nitroimidazoles Delamanid and Pretomanid inhibit at mycobacteria the formation of mycolic acids which constitute cell wall components.
Classification according to the mechanism of action
Inhibition of cell wall synthesis
-
β-lactam antibiotics :
- Carbapenems : Imipenem , Meropenem , Ertapenem
- Cephalosporins
- Monobactams : aztreonam
- Penicillins : Penicillin G (Benzylpenicillin), Penicillin V ( Phenoxymethylpenicillin )
- β-lactamase inhibitors : clavulanic acid , sulbactam , tazobactam
- Sultamicillin
- Nucleoside antibiotics , e.g. B. Puromycin , Blasticidin S , Polyoxine
- Epoxy Antibiotics : Fosfomycin
- Glycopeptides : teicoplanin , vancomycin
- Polypeptides : Bacitracin , Colistin , Gramicidin , Polymyxin B , Tyrothricin , Teixobactin
- Fosmidomycin
- Bicyclic nitroimidazoles : Pretomanid , Delamanid
Inhibition of protein synthesis on the ribosome
- Aminoglycosides : Amikacin , Gentamicin , Kanamycin , Neomycin , Netilmicin , Streptomycin , Tobramycin
- Chloramphenicol
- Fusidic acid
- Ketolides : Cethromycin , narbomycin , telithromycin
- Lincosamides : clindamycin , lincomycin
- Lipopeptides : daptomycin , caspofungin
- Streptograms : Dalfopristin , Quinupristin
- Macrolides : Azithromycin , Clarithromycin , Erythromycin , Roxithromycin
- Oxazolidinone : Cycloserine (now called Terizidone ), Linezolid , Tedizolid , Eperezolid
- Tetracyclines : Doxycycline , Minocycline , Tetracycline , Oxytetracycline
- Glycylcycline : tigecycline
Effect on bacterial nucleic acids
- Gyrase inhibitors (inhibitors of DNA replication)
- Generation 1: norfloxacin , enoxacin
- Generation 2: ciprofloxacin , ofloxacin
- Generation 3: Levofloxacin
- Generation 4: moxifloxacin
- Bactericidal DNA damage (complex formation, strand breakage)
- 2- and 5- nitroimidazoles : metronidazole , tinidazole , nimorazole
- Folic acid antagonists
- Sulfonamides : sulfadiazine , sulfadoxine , sulfamethoxazole , sulfasalazine
- Diaminopyrimidines : pyrimethamine , trimethoprim
- Ansamycins (inhibitors of bacterial RNA polymerase)
Inhibition of the energy metabolism
Problems
Side effects
As a rule, antibiotics are well tolerated and have a wide therapeutic range . Side effects are primarily allergies , disruption of the intestinal flora ( antibiotic-associated diarrhea ) and the occurrence of fungal infections , rarely pseudomembranous colitis . When treating with broad spectrum antibiotics, the disruption or destruction of the intestinal flora can trigger the life-threatening infection with Clostridium difficile . In particular, a strong and constant treatment can lead to permanent or permanent damage to the microorganisms colonizing the intestine. The intestinal flora of children up to around the age of three is particularly sensitive to antibiotics, as it is during this time that they have their crucial developmental phase.
Fluoroquinolones from the group of gyrase inhibitors can lead to tendon tears and other previously unknown side effects. There is still a need for research on this.
Antibiotics have been linked to kidney stones . The incidence is particularly high in children.
Another undesirable effect (e.g. of erythromycin , cotrimoxazole , pentamidine and moxifloxacin ) can be the prolongation of the QTc time , which is visible in the ECG, with the resulting cardiac arrhythmia .
Many antibiotics are associated with neurotoxic side effects such as hearing damage, neuropathies or encephalopathies ( aminoglycosides , cephalosporins , penicillins , carbapenems , tetracyclines , sulfonamides , macrolides , fluoroquinolones , oxazolidinones , polymyxins , clindamantycin , vancomurycin and nitr ). Antibiotics rarely cause other organ-toxic effects, such as kidney damage from gentamicin and polymyxins. Some antibiotics such as bacitracin or colistin show such strong side effects when administered systemically (internally) that they are only applied topically. In this case one speaks of local antibiotics. In some infections such as syphilis or borreliosis , antibiotics can trigger a so-called Herxheimer reaction , in which the organism is flooded with toxins from killed bacteria.
Side effects are often temporary, but in rare cases they can lead to long-term damage and permanent disabilities. Fluoroquinolones ( ofloxacin , ciprofloxacin , levofloxacin , moxifloxacin ) cause the majority of permanent disabilities, but other antibiotics such as cefdinir , nitrofurantoin , amoxicillin , doxycycline , amoxicillin-clavulanate , clarithromycin , cotrimoxazole , cephalexin and azithromycin can leave permanent damage.
Side effects can be avoided by restricting excessive prescription, e.g. B. in viral diseases, and a delayed prescription can be reduced. With delayed prescription, prescriptions are only redeemed in the event of deterioration, since spontaneous improvement is possible within the following days. In the case of various diseases, a shorter antibiotic therapy with constant treatment success is possible, which lowers the risk of side effects. In the case of pneumonia 3–5 instead of 7–10 days, intra-abdominal infections 4 instead of 10 days, acute bacterial sinuitis 5 instead of 10 days and chronic bone marrow inflammation 42 instead of 84 days.
Interactions
Many antibiotics can influence the effects of other drugs or luxury foods. For example, some gyrase inhibitors can prevent the liver from breaking down the stimulant caffeine ; the interaction may be clinically relevant. High levels of caffeine can cause palpitations, headache, and dizziness . Different antibiotics of the tetracycline group lose their effectiveness in combination with milk, cheese, quark and yoghurt. They are inactivated by the calcium ions (Ca 2+ ) contained in milk and milk products through complex formation .
Resistance and the development of new antibiotics
Under antibiotic resistance is defined as the resistance of bacteria to antibiotics. The proliferation of individual bacteria that are not killed by antibiotics or their growth is inhibited, results in resistant bacterial strains, the treatment of which with a specific or even several antibiotics is ineffective.
Multi-resistant bacteria are now increasingly appearing (see MRSA ). The first “super-pathogens” that are resistant to all known antibiotics have already been identified. It is a growing problem: in 2005 around three million Europeans became infected with bacteria resistant to known antibiotics - 50,000 of whom died from it; In Africa in particular , people die from infections with diseases that cannot be treated with antibiotics. If one does not steer against it on all fronts, an estimated 10 million people are likely to die of such resistant pathogens around the year 2050.
In severe cases, “ reserve antibiotics ” are used, which should not be used for normal therapies. These currently include linezolid , daptomycin , tigecycline and streptograms (especially quinupristin and dalfopristin ). The World Health Organization (WHO) warned in 2012 that mutants of gonorrhea could soon become resistant to a broad spectrum antibiotic that is considered the last possible treatment option.
The main reason for the increasing resistance is the increased use of antibiotics.
In addition to the already known for several years resistance of gram-positive bacteria ( methicillin-resistant Staphylococcus aureus , MRSA, vancomycin-resistant Staphylococcus aureus , VRSA, vancomycin-resistant enterococci , VRE) are recently increasingly resistance in Gram-negative pathogens to about β-lactam Antibiotics ( NDM-1- forming bacteria) known as MRGN (multi-resistant gram-negative bacteria) have been observed . Multi-drug resistance (MDR) in mycobacteria , the causative agents of tuberculosis, which is still widespread, are also problematic . The global mobility of humans and animals promotes the spread of resistance.
The problem of antibiotic resistance makes it necessary to continuously develop new antibiotics with which the resistant pathogens can be inhibited from growing. However, the number of new antibiotics coming onto the market every year is falling continuously. This is to be regarded as questionable, since, on the other hand, antibiotic resistance is constantly increasing. The decline in new antibiotics coming onto the market is attributed to a market failure . Since new antibiotics are typically initially held in reserve, no short-term profit can be made from them. On the other hand, the development through the necessary approval procedures, but also through the start-up of production, requires high financial outlay. Due to an increased focus on increasing the shareholder value in the pharmaceutical industry, as of 2019, almost all large pharmaceutical groups stopped their advance development in this area due to a lack of profit prospects. Leading experts in the field of antibiotic research rate the situation as extremely alarming and warn of serious future consequences, such as a sharp increase in deaths due to untreatable infections. The problem of multi-resistant germs is exacerbated by the producers of antibiotics, who partially allow residues from production to flow unfiltered into the waters of the environment, such as in Hyderabad in India. In 2017, German scientists took dozens of samples there for the presence of multi-resistant germs and analyzed them in the laboratory in Germany. An unusually high number of these dangerous bacteria was found in the natural waters and in the water system in large parts of Hyderabad.
Special fields of application
livestock farming
Antibiotics are also used in animal husbandry . A distinction must be made between two different uses: on the one hand, as a drug that is specifically used in the context of veterinary treatment. On the other hand, the use of antibiotics as growth and performance enhancers , which is particularly controversial. This latter type of use was banned in the EU at the beginning of 2006 after it was no longer allowed to be used in Denmark in 1995, in Vorarlberg since 1997 and in Switzerland in 1999 due to national self-restrictions.
If a single animal has a bacterial infection, veterinary treatment may require antibiotic treatment of the entire herd. In this application, called metaphylaxis , a particularly high selection pressure is created on the bacterial strains present in the stables, which allows only the few resistant pathogens ( normally present through natural mutation ) to survive. However, all sensitive microorganisms are killed. The remaining pathogens then form the resistant strain if they are not killed as a residual infection by the immune reaction of the animal or human. This can make the antibiotic ineffective against the known infections. Resistant bacteria can then reach other organisms and lead to more difficult disease progression or even therapy failure. This has resulted in increased resistance to antibiotics in animals and humans in the past. Workers in pig and poultry farms are mainly at risk. In the United States, it is estimated that animals are given at least the same amount of antibiotics as humans. Antibiotic-resistant Salmonella , Campylobacter and Escherichia choli strains, which are pathogenic to humans, are being detected with increasing frequency in large poultry and cattle farms.
Molecular Biology Research
Antibiotics are also used as selection agents in molecular biology . When cloning , the property of resistance to a certain antibiotic is used as an indicator of whether a strain carries a certain gene that one would like to incorporate into the bacterium. Both the new gene and the resistance information are located on a plasmid . The bacterium is propagated on a medium that contains the appropriate antibiotic. This also signals a subsequent loss of the plasmid, since if it is lost, the resistance is also lost and the bacterium on the medium dies.
Consumption statistics
Since 1992, the classic β-lactams (penicillins, aminopenicillins and cephalosporins) have expanded their leading position measured in defined daily doses , while the proportion of tetracyclines has decreased. Aminopenicillins (mainly amoxicillin) have practically replaced the oral penicillins.
Research and Alternatives
In addition to the further development of known classes of substances with known active principles, which make up the majority of the market launches in the last few decades, the development of substances with novel points of attack is also seen as necessary. In addition to the previously predominant targets (cell wall synthesis, ribosomal protein synthesis, DNA replication, folic acid synthesis), for example
- at the amino acid synthesis enzymes involved
- the bacterial fatty acid biosynthesis
- Proteins that play a role in the interaction between host and pathogen
- Proteins that contribute to the persistence of bacteria or to their survival
become increasingly important as additional points of attack. The expansion of the search for new molecules to include ecological niches and the genomes of microorganisms, especially not cultivated ones ( metagenome ), is also gaining in importance. An innovative strategy is the inhibition of pathogenic germs without killing them. This eliminates the selection pressure that contributes to the spread of resistance.
Some new substances or substance groups and active principles have been described in the recent past from preclinical research: plectasin, for example, which comes from the group of so-called defensins that are common in fungi, animals and plants and not only disrupts bacterial cell wall synthesis, but also the immune system of the Intended to stimulate the host; Platensimycin and Platencin , two substances isolated from the soil bacterium Streptomyces platensis that selectively inhibit bacterial lipid biosynthesis; the novel RNA polymerase inhibitors myxopyronin, corallopyronin, and ripostatin; the MTAN inhibitors, which intervene in quorum sensing and suppress the ability of certain bacteria to form protective biofilms ; Closthioamide, a structurally unusual molecule with numerous sulfur atoms, which is formed by the anaerobic soil bacterium Clostridium cellulolyticum and is active against multi-resistant pathogens; the triclosan derivative PT70, which inhibits mycolic acid synthesis in mycobacteria.
Only recently discovered and antibiotic active acyldepsipeptides (ADEPs) are in the phase of basic research . They override a special control function in bacterial cells, which is a novel mechanism of action. The novel active ingredients have as a target a ClpP - protease , a protein-zerschneidendes enzyme . Normally, this special protease effects the recycling of defective bacterial proteins through a strictly controlled process . If this control process is suppressed by ADEPs, the ClpP protease also breaks down healthy proteins that are vital for the metabolism of the bacterium, including the FtsZ protein , which is important for cell division . The bacteria can no longer divide and die. With the new group of active ingredients, ADEPs, the researchers hope to find a new, broadly effective antibiotic that would also be able to kill multi-resistant bacteria.
Researchers at the Christian-Albrechts-Universität zu Kiel have discovered that the freshwater polyp Hydra magnipapillata produces a protein called hydramacin-1 , which literally clumps and kills a number of bacteria. They also succeeded in isolating the associated gene so that they could produce the protein in its pure form for further studies. They found that hydramacin-1 can kill enterobacteria , klebsiae , streptococci and yersinia even in relatively small doses . However, it was less effective against some other germs such as Staphylococcus aureus .
Treatment with bacteriophages - highly specialized viruses that bacteria use as host cells - also promises help in the event of infections with resistant bacteria . Various pharmaceutical companies are currently working on the approval of phage therapy .
Teixobactin from the soil bacterium Eleftheria terrae , which was presented in an article in the journal Nature in January 2015, represents a new group of substances . The substance teixobactin, which is effective against Gram- positive bacteria , has not yet shown any resistance development in those bacteria that are sensitive to teixobactin, because the structure of the bacterial cell wall is interfered with "at many critical points" and resistance formation is made more difficult by many simultaneously necessary adaptations of the bacteria .
Another group among the new types of antibiotics are the Outer Membrane Protein Targeting Antibiotics (abbreviated "Ompta") , which are effective against gram-negative germs . They interact with components of the outer membrane of gram-negative bacteria, on the one hand by binding to fat-like membrane components and on the other hand to the membrane protein Bam A. This is important for the structure of the outer shell of the bacteria. Because the membrane can no longer be formed, the germs die. Models for synthetic ompta are natural antimicrobial proteins such as the protegrin found in pigs or the thanatin from the bug. A first candidate, Murepavadin , was in the clinical trial against respiratory infections caused by Pseudomonas aeruginos until 2019 , and another substance (POL7306) is to be transferred to clinical trials in humans in 2020.
criticism
Antibiotic abuse
The effectiveness of antibiotics is fundamentally beyond question and in many cases is life-saving. The organized use of antibiotics to prevent disease and improve performance in animal fattening is rejected by doctors. The use of antibiotics for viral infections and inflammation of the upper respiratory tract or, for example, the paranasal sinuses is usually pointless due to the ineffectiveness of antibiotics against viruses (one exception is the research chemical feglymycin ) and can increasingly contribute to the development of resistance in bacteria. For these reasons, the indication for each antibiotic therapy must be decided individually and responsibly. This is also known as “ Antibiotic Stewardship ” in the English-speaking world .
Residues in the environment
Antibiotic-resistant bacteria are released in large quantities directly into the environment via liquid manure and manure spreading from intensive animal husbandry . In addition, antibiotics themselves are introduced into the environment through direct substance input. There they develop a biological effect and could also cause an increase in antibiotic-resistant bacteria there. Recent studies show a sharp increase in multi-resistant bacteria in the environment. Resistant pathogens can return to humans wherever there is contact with faecal polluted water such as bathing water. Scientists are calling for antibiotics from animal husbandry to be reduced.
literature
- Ursula Theuretzbacher: Microbiology in everyday clinical practice. Pathogens, diagnostics, therapy. 2nd Edition. Kohlhammer, Stuttgart 1999/2005, ISBN 3-17-016665-4 .
- Claus Simon, Wolfgang Stille: Antibiotic therapy in clinics and practices. Schattauer, Stuttgart 1985, ISBN 3-7945-1970-1 .
- Wolfgang Stille, Hans-Reinhard Brodt, Andreas H. Groll, Gudrun Just-Nübling: Antibiotic Therapy. 1st reprint of the 11th edition, Schattauer, Stuttgart 2006, ISBN 3-7945-2160-9 .
- Peter Heisig: What's new about ketolides and oxazolidinones? Mechanisms of action and resistance. In: Pharmacy in our time . Volume 33, No. 1, 2004, pp. 10-19, ISSN 0048-3664 .
- Radka Alexy, Klaus Kümmerer: Antibiotics in the environment. In: KA: Correspondence waste water, waste. Vol. 52, No. 5, 2005, ISSN 1616-430X , pp. 563-571.
- Franz Daschner , Uwe Frank, Evelina Tacconelli: Antibiotics at the bedside (= 1x1 of the therapy. ) 16th, completely revised and updated edition, Springer Berlin / Heidelberg / New York 2013, ISBN 978-3-642-25678-3 .
- Uwe Frank, Winfried Ebner, Franz Daschner : Antibiotics in Practice 2019-2020: with hygiene advice. 10th, completely revised and updated edition, Springer-Verlag, Berlin 2019, ISBN 978-3-642-25626-4 .
- M. Grote, C. Schwake-Anduschus, H. Stevens, R. Michel, T. Betsche, M. Freitag: Antibiotic uptake of crops from manure-fertilized soils - results of a model experiment. In: Journal for Consumer Protection and Food Safety. Volume 1, No. 1, Birkhäuser, Basel 2006, ISSN 1661-5751 , pp. 1661-5867.
- Hidetada Hirakawa, Haruyoshi Tomita: Interference of bacterial cell-to-cell communication: A new concept of antimicrobial chemotherapy breaks antibiotic resistance. In: Frontiers in Microbiology. No. 4, May 13, 2013, p. 114, doi: 10.3389 / fmicb.2013.00114 .
- Ulrike Roll: Antibiotics. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 68 f.
- Ulrike Holzgrabe : How good are the new antibiotics? . In: Pharmaceutical newspaper . Issue 21/2018 from May 23, 2018.
Web links
- CT Walsh, MA Fischbach: Antibiotics: New strategies against super germs . Spectrum of Science 4/10 (April 2010). can be viewed online at schattenblick.de.
- BURDEN of Resistance and Disease in European Nations - Determining the Financial Burden of Antibiotic Resistance in Europe
- SARI - Surveillance of antibiotic use and bacterial resistance in intensive care units
Individual evidence
- ^ William Martindale, EF James Reynolds et al .: The Extra Pharmacopoeia. 29th edition. Pharmaceutical Press, London 1989, ISBN 0-85369-210-6 , p. 94.
- ^ A b c E. Mutschler, G. Geisslinger, HK Kroemer, P. Ruth, M. Schäfer-Korting: drug effects . Textbook of pharmacology and toxicology. 9th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 , p. 794.
- ^ Roche-online Lexicon Medicine
- ↑ after: W. Köhler, HJ Eggers, B. Fleischer: Medical Microbiology. 8th edition. Elsevier, Munich 2001, p. 200.
- ↑ J.-M. Schröder: The body's own antibiotics protect the skin and mucous membranes . In: Pharmaceutical newspaper. Issue 16, April 19, 2010.
- ↑ Mildred Savage: Elixirs of Life. Translated from the American by Margitta de Hervás, Scherz, Bern.
- ↑ Ulrike Roll: Antibiotics. Berlin / New York 2005, p. 68.
- ↑ Freya Harrison et al .: A 1,000-Year-Old Antimicrobial Remedy with Antistaphylococcal Activity . mBio 6 (4): e01129-15. American Society for Microbiology, August 15, 2015, doi : 10.1128 / mBio.01129-15 .
- ↑ On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ. A. Fleming, Br J Exp Pathol 10 (3): pp. 226-236. (1929). PMC 2048009 (free full text)
- ↑ Christof Goddemeier: Alexander Fleming (1881–1955): Penicillin. In: Deutsches Ärzteblatt . Volume 103, No. 36, 2006, p. A2286. Retrieved February 3, 2017.
- ↑ Franz von Nussbaum, Michael Brands, Berthold Hinzen, Stefan Weigand, Dieter Häbich: Antibacterial Natural Products in Medicinal Chemistry - Exodus or Renaissance? In: Angewandte Chemie . tape 118 , no. 31 , August 2006, p. 5194-5254 , doi : 10.1002 / anie.200600350 .
- ^ Franz von Nussbaum, Michael Brands, Berthold Hinzen, Stefan Weigand, Dieter Häbich: Antibacterial Natural Products in Medicinal Chemistry — Exodus or Revival? In: Angewandte Chemie International Edition . tape 45 , no. 31 , August 2006, p. 5072-5129 , doi : 10.1002 / anie.200600350 .
- ↑ Herbert Hof, Rüdiger Dörries: Medical Microbiology (= dual series ). Thieme, Stuttgart 2014, ISBN 978-3-13-125315-6 , p. 302.
- ^ William Barry Hugo, SP Denyer, Norman A. Hodges, SP Gorman: Hugo and Russell's pharmaceutical microbiology. John Wiley & Sons, Malden MASS 2004, ISBN 0-632-06467-6 , p. 205, (online)
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 335-337.
- ↑ E. Mutschler, G. Geisslinger, HK Kroemer, M. Schäfer-Korting: drug effects. 8th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2001, ISBN 3-8047-1763-2 . P. 820 f.
- ↑ G. Geisslinger et al .: Mutschler drug effects . 11th edition. WVG, Stuttgart 2019, p. 1048.
- ↑ G. Geisslinger et al .: Mutschler drug effects . 11th edition. WVG, Stuttgart 2019, p. 1047.
- ↑ Unwanted side effects - antibiotic therapies damage the intestinal flora for months. Accessed December 16, 2018 (German).
- ↑ Deutschlandfunk: When hospital germs have an exit - resistant pathogens leave the clinic . dated November 2, 2009.
- ↑ Joël Doré ( INSA ): The effect of intestinal bacteria goes beyond the digestive tract ( Memento from June 18, 2012 in the Internet Archive ). From arte.tv , June 11, 2012, accessed June 16, 2012.
- ↑ Deutsche Apothekerzeitung : How toxic are the gyrase inhibitors? , Stuttgart, December 22, 2015.
- ↑ Ralf Stahlmann and Hartmut Lode: Side Effects of the Newer Fluoroquinolones ( Memento from August 1, 2014 in the Internet Archive ), Paul Ehrlich Institute , Berlin
- ↑ Gregory E. Tasian, Thomas Jemielita, David S. Goldfarb, Lawrence Copelovitch, Jeffrey S. Gerber, Qufei Wu, Michelle R. Denburg: Oral Antibiotic Exposure and Kidney Stone Disease. In: Journal of the American Society of Nephrology , S. ASN.2017111213, doi : 10.1681 / ASN.2017111213 .
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, p. 512.
- ↑ Neurotoxicity of antibiotics . In: Journal of Chemotherapy . tape 36 , no. 5 , p. 41-44 ( infektio.de [PDF]).
- ^ Marie F Grill, Rama K Maganti: Neurotoxic effects associated with antibiotic use: management considerations . In: British Journal of Clinical Pharmacology . tape 72 , no. 3 , September 2011, PMC 3175508 (free full text).
- ↑ FDA: Fluoroquinolone Safety Labeling Changes. In: FDA. FDA, April 2017, accessed January 19, 2019 .
- ↑ Carl Llor, Lars Bjerrum: Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem . In: Therapeutic Advances in Drug Safety . tape 5 , no. December 6 , 2014, PMC 4232501 (free full text).
- ^ Brad Spellberg: The New Antibiotic Mantra— "Shorter Is Better" . In: JAMA internal medicine . tape 176 , no. 9 , September 1, 2016, PMC 5233409 (free full text).
- ↑ H. Schneemann et al. (Ed.): Applied drug therapy - clinical-pharmaceutical care in case studies . Springer Verlag, 2001, p. 689.
- ↑ Grit Ackermann (Ed.): Antibiotics and antimycotics, substances - clinical pictures - pathogen-specific therapy. Medical-pharmacological compendium. 4th edition. Volume 8, Deutscher Apotheker Verlag, 2013, p. 113.
- ^ Katrin Hoerner: Interactions: Unwanted Influence. - Milk, yogurt, cheese interactions with antibiotics . On: focus.de , accessed on June 8, 2015.
- ↑ Risk food: interactions with drugs . On: Gesundheit.de , updated: May 25, 2012, accessed on June 8, 2015.
- ↑ Wolfgang Stieler: Doctors warn of a "post-antibiotic age" . On: heise.de , March 30, 2007; last accessed on March 2, 2016.
- ↑ Ursula Biermann: Antibiotic resistance - 250 people die every minute. On: deutschlandfunk.de from May 24, 2016, last accessed on July 30, 2017.
- ↑ No antibiotics for healthy animals. Neue Zürcher Zeitung , November 9, 2017, accessed on November 10, 2017.
- ↑ Magdalena Schmude: WHO list - which antibiotics should only be used little . On: deutschlandfunk.de from June 12, 2017.
- ↑ Scientific news from July 20, 2017 [AUDIO]: see audio file: Selection compact: 'Antibiotika Overkill' - The attack on our immune system. On: deutschlandfunk.de from July 20, 2017.
- ↑ WHO concerned about gonorrhea - clap pathogens increasingly resistant to drugs. On: sueddeutsche.de (Süddeutsche Zeitung) from June 6, 2012.
- ↑ Deutschlandfunk; News from June 7th 2012: Microbiology - gonorrhea could become incurable . On: deutschlandfunk.de ; last accessed on July 30, 2017.
- ↑ PHE press office: New report reveals increase in use of antibiotics linked to rising levels of antibiotic resistance. Retrieved January 17, 2019 .
- ↑ U. Holzgrabe, J. Schmitz: New antibiotics. Maintain the lead. In: Pharmaceutical newspaper. Edition 50/2009, online version .
- ↑ NDR: Deadly Danger: The End of Antibiotics? Retrieved September 15, 2019 .
- ↑ [1] , October 22, 2019, by C. Baars, E. Kuch, C. Adelhardt and B. von der Heide, NDR.
- ↑ [2] , The Invisible Enemy - Tödliche Supererreger aus Pharmafabriken, video documentation, Germany, 2017, by Karin Bauer and Christian Baars, English subtitle, 45 min., The story in the first , on youtube [3]
- ↑ Dangerous super germs from India in Basel wastewater , by Urs P. Gasche, June 20, 2019.
- ↑ Food safety and food intolerance ( Memento of February 26, 2011 in the Internet Archive ), European Food Information Center (EUFIC).
- ↑ SR 910.1 Federal Law on Agriculture, Art. 160, Para. 8 .
- ↑ M. Gilchrist, C. Greko, D. Wallinga, G. Beran, D. Riley, P. Thorne: The Potential Role of Concentrated Animal Feeding Operations in Infectious Disease Epidemics and Antibiotic Resistance. In: Environmental Health Perspectives . February 2007, Volume 115, Number 2, pp. 313-316, doi: 10.1289 / ehp.8837 .
- ↑ David Tilman, Kenneth G. Cassman, Pamela A. Matson, Rosamond Naylor, Stephen Polasky: Agricultural sustainability and intensive production practices. In: Nature . No. 418, August 8, 2002, pp. 671-677, doi: 10.1038 / nature01014 .
-
↑ Ulrich Schwabe et al .: Drug Prescription Report 2002. Current data, costs, trends and comments. Springer, Berlin 2003, ISBN 3-540-43624-3 , p. 132;
Ulrich Schwabe, Dieter Paffrath, M. Anlauf a. a .: Drug Prescription Report 2010. Current data, costs, trends and comments. E-book. Springer, Berlin 2010, ISBN 978-3-642-13380-0 , p. 304. - ↑ Novozymes reveals knowledge on new antibiotic against resistant bacteria. (No longer available online.) In: Novozymes. May 28, 2010, archived from the original on November 14, 2012 ; Retrieved June 27, 2011 .
- ↑ Per H. Mygind1, Rikke L. Fischer et al .: Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungu. In: Nature. Volume 437, number 7061, 2005, pp. 975-980, doi: 10.1038 / nature04051 .
- ↑ Plectasin NZ2114 - Novel Microbial Agent. In: Drug Development Technology. Retrieved May 28, 2010 .
- ↑ Novel antibiotics outsmart resistance ( Memento of December 11, 2012 in the Internet Archive ). On: biotechnologie.de , October 22, 2008.
- ↑ New antibiotics: bacteria without a protective shield . In: Pharmaceutical newspaper. PZ-Nachrichten, March 17, 2009.
- ↑ New antibiotic discovered against multi-resistant germs. In: vetline.de. April 22, 2010, archived from the original on February 12, 2013 ; Retrieved August 19, 2016 .
- ↑ Highly effective inhibitor keeps tuberculosis in check ( Memento of December 11, 2012 in the Internet Archive ). On: biotechnologie.de , June 28, 2010.
- ↑ Peter Sass et al .: Antibiotic acyldepsipeptides activate ClpP peptidase to degrade the cell division protein FtsZ. In: Proceedings of the National Academy of Sciences. Volume 108, number 42, 2011, pp. 17474-17479, doi: 10.1073 / pnas.1110385108 .
- ↑ Sascha Jung: The elucidation of the tertiary structure of the antimicrobial peptide Hydramacin-1 with the help of multi-dimensional heteronuclear NMR spectroscopy and the investigation of its mechanism of action. Dissertation, Christian-Albrechts-Universität zu Kiel - Faculty of Mathematics and Natural Sciences, Kiel 2008 ( PDF file ).
- ^ Sascha Jung, Andrew J. Dingley, René Augustin et al .: Activity of a Protein from the Basal Metazoan Hydra. In: Journal of Biological Chemistry. Volume 284, number 3, pp. 1896-1905, doi: 10.1074 / jbc.M804713200 ( full text online ).
- ↑ Thomas CG Boscha, René Augustina, Friederike Anton-Erxleben a. a .: Uncovering the evolutionary history of innate immunity: The simple metazoan Hydra uses epithelial cells for host defense. In: Developmental & Comparative Immunology. Volume 33, Number 4, April 2009, pp. 559-569, doi: 10.1016 / j.dci.2008.10.004 .
- ^ Dietrich von Richthofen: Alternative to antibiotics: Doctors send viruses on a deadly mission . On: handelsblatt.com , August 26, 2008; last accessed on May 10, 2014.
- ↑ LL Ling, T. Schneider, AJ Peoples et al .: A new antibiotic kills pathogens without detectable resistance. In: Nature. [electronic publication before printing] January 2015, doi: 10.1038 / nature14098 . PMID 25561178 .
- ↑ A. Mende: New approach against gram-negative bacteria , PZ, November 15, 2018.
- ↑ Shortly before the goal , Laborjournal, July 25, 2019.
- ^ DGAP news , July 17, 2019.
- ↑ Researchers discover antibiotics with novel effects . On: NZZ.ch , October 23, 2019; last accessed on October 25, 2019.
- ^ I. Feuerpfeil, J. López-Pila, R. Schmidt, E. Schneider, R. Szewzyk: Antibiotic-resistant bacteria and antibiotics in the environment . In: Federal Health Gazette . tape 42 , no. 1 , January 1999, p. 37-50 , doi : 10.1007 / s001030050057 .