Chloramphenicol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
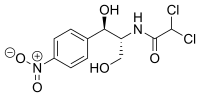
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Chloramphenicol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 12 Cl 2 N 2 O 5 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 323.14 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.7 g cm −3 |
||||||||||||||||||
| Melting point |
150 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chloramphenicol is a broad-spectrum antibiotic that was first obtained in 1947 from Streptomyces venezuelae by two research groups under Paul R. Burkholder (University of Yale) and David Gottlieb (1911–1982) .
Due to the potentially life-threatening aplastic anemia which occurs in rare cases (≈ 3–17 out of 100,000) as a side effect, chloramphenicol should only be used as a reserve antibiotic after careful consideration . Although topical use was discouraged as early as 1985 , in Western Europe it can still be found in eye and ear drops, in eye ointments and skin medicines .
The main areas of treatment are severe, otherwise uncontrollable infectious diseases such as typhus , paratyphoid , plague , typhus , dysentery , diphtheria and malaria . In addition, chloramphenicol is effective against chytridiomycosis , a fatal and highly contagious fungal skin disease for amphibians , which is decimating amphibian populations worldwide.
Chloramphenicol is now produced exclusively using fully synthetic methods.
pharmacology
Chloramphenicol is a translation inhibitor , so it has a blocking effect on the formation of the peptide bond by reversibly binding to the catalytic center of the peptidyl transferase of the 50S subunit of the bacterial 70S ribosomes and inhibiting them. It is a bacteriostatic antibiotic. It is easily accessible to tissue, including through the placenta and into breast milk. The good tissue penetration is one of the reasons why it is used externally and locally on the eye as an ointment, for example for infections of the eyelid (e.g. stye ).
Pharmacokinetics
Chloramphenicol is quickly and completely absorbed after oral administration. The bioavailability is 80% after oral administration and 70% after intramuscular injection. The plasma protein binding is between 50% and 60%. The plasma half-life is 1.5–3.5 hours in adults with normal liver and kidney function, 3–6.5 hours in children and adolescents, and 24 hours or longer in newborns. 90% of the elimination takes place via conjugation to glucuronic acid . In adults with impaired renal function, the plasma half-life is extended to 3–4 hours, and in severe liver dysfunction to 4.6–11.6 hours.
Side effects and interactions
- Bone marrow damage: dose-dependent, reversible - disorder of erythropoiesis ; Irrespective of the dose, irreversible bone marrow aplasia ( aplastic anemia ) can be triggered, with a delay of 2–8 weeks
- Grey's Syndrome
- neurotoxic
- Contact sensitization with topical application
- allergic reactions ; anaphylactic reactions and generalized urticaria when used systemically
- Herxheimer reaction
- Interactions with oral anticoagulants, methotrexate , sulfonylureas - in the sense of an increase in effectiveness
- Barbiturates and phenytoin reduce the effects of chloramphenicol
- simultaneous use of oral contraceptives can impair their effectiveness.
The risks of topical- dermal use are controversial. Representatives of the manufacturer of Ichtoseptal point out that their literature research did not reveal any evidence of damage to the blood-forming organs after topical dermal application of chloramphenicol. Critics of topical-dermal application point out that chloramphenicol is always absorbed percutaneously . The rare (1: 25,000 - 1: 50,000), mostly fatal, dose-independent, irreversible form of aplastic anemia has also been observed after topical application, regardless of its duration. Aplastic anemia can occur even months after stopping the medication. Among all drugs, chloramphenicol is the active ingredient that is most often blamed for aplastic anemia. In the absence of systematic, long-term blood counts, no statement can be made about the true incidence of haematological side effects . It is assumed that the development of drug-induced aplastic anemia is due to the generation of metabolites with reactive nitroso groups that are able to damage the DNA.
Contraindications
Chloramphenicol is contraindicated, especially in newborns, due to its bone marrow depressive effect and the limited therapeutic range ( Grey's syndrome ). It is also contraindicated in severe hepatic insufficiency, pregnancy and lactation.
The use of chloramphenicol in food-producing animals is generally prohibited in the European Union in accordance with the EU maximum residue limit regulation for food of animal origin .
microbiology
The antibiotic effect of chloramphenicol is used in microbiology to inhibit bacterial growth. For example, most yeasts can grow on a malt extract medium with chloramphenicol, but bacterial growth is effectively inhibited. In addition, chloramphenicol is now also used to improve FISH (fluorescence in situ hybridization), where protein synthesis and the effect of inhibiting the breakdown of rRNA are used.
Some organisms can inactivate chloramphenicol with a specific acetyltransferase by introducing an acetyl group on each of the two hydroxyl groups. This renders the antibiotic ineffective and no longer binds to ribosomes.

Trade names
Halomycetin (A), Kemicetin (A), Posifenicol (D), Septicol (CH) and one generic (A)
Aquapred (D), Ichtoseptal (D), Sperdex comp. (CH)
Chloro-Sleecol, Chloromycetin, Otiprin N, Prurivet S
literature
The frequency of occurrence of aplastic anemia:
- K. Hausmann, G. Skrandies: Aplastic Anemia following chloramphenicol therapy in Hamburg and surrounding districts. In: Postgrad Med J. 50 (Suppl.) 1974, pp. 131-136.
- K. Hausmann, G. Skrandies, P. Sachtleben: Current aspects of drug-related bone marrow damage. In: Münch Med WSchrft. 116, 1974, pp. 1621-1626.
Individual evidence
- ↑ a b c d e f Entry on chloramphenicol in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c Entry on chloramphenicol. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ CliniPharm: drug data .
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid 1961, pp. 9-223, here: pp. 51 f.
- ↑ H. Kiffe, H. Ippen: Systemic side effects from external use of chloramphenicol. In: The dermatologist. 36, Springer-Verlag 1985, pp. 181-183.
- ↑ Birte Vester, Andrea T. Feßler, Geovana Brenner Michael, Yang Wang, Kristina Kadlec: Lincosamides, Streptogramins, Phenicols, and Pleuromutilins: Mode of Action and Mechanisms of Resistance . In: Cold Spring Harbor Perspectives in Medicine . tape 6 , no. 11 , November 1, 2016, p. a027037 , doi : 10.1101 / cshperspect.a027037 , PMID 27549310 , PMC 5088508 (free full text) - ( cshlp.org [accessed February 6, 2019]).
- ↑ Amanda Y. Shen, Elie J. Haddad, David J. Hunter-Smith, Warren M. Rozen: Efficacy and adverse effects of topical chloramphenicol ointment use for surgical wounds: a systematic review . In: ANZ Journal of Surgery . tape 88 , no. 12 , 2018, p. 1243-1246 , doi : 10.1111 / ans.14465 .
- ^ J. Warnecke, W. Fehrs (ICHTHYOL-Gesellschaft Hamburg): Statement on the work of PH Höger: "Topical antibiotics and antiseptics"; Dermatologist (1998) 49: 331-347. In: Der Hautarzt 12, 98, Springer-Verlag 1998, p. 938. doi: 10.1007 / s001050050853 .
- ^ PH Höger: Statement by the author. In: The dermatologist. 12, 98, Springer-Verlag 1998, p. 939.
- ↑ Shiho Ohnishi, Mariko Murata, Naoyuki Ida, Shinji Oikawa, Shosuke Kawanishi: Oxidative DNA damage induced by metabolites of chloramphenicol, an antibiotic drug . In: Free Radical Research . tape 49 , no. 9 , July 2015, doi : 10.3109 / 10715762.2015.1050963 , PMID 25971446 .
- ^ JA Turon, CM Andrews, AC Havard, TC Williams: Studies on the haemotoxicity of chloramphenicol succinate in the Dunkin Hartley guinea pig . In: International Journal of Experimental Pathology . tape 53 , no. 5 , PMID 12641819 .
- ^ Theodor Dingermann, Rudolf Hänsel, Ilse Zündorf (eds.): Pharmaceutical Biology: Molecular Basics and Clinical Applications. 1st edition. Springer Verlag, Berlin 2002, ISBN 3-540-42844-5 , p. 301.
- ↑ Red List Online, as of August 2009.
- ↑ AM comp. d. Switzerland, as of August 2009.
- ↑ AGES-PharmMed, as of August 2009.