Streptomycin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
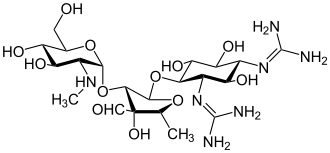
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Streptomycin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 39 N 7 O 12 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Protein synthesis inhibitor |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 581.57 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Streptomycin is an aminoglycoside - antibiotic , which by many Streptomyces is formed.
history
Streptomycin was first isolated from Streptomyces griseus on October 19, 1943 by Selman Waksman and his colleagues Albert Schatz and Elizabeth Bugie at Rutgers University . For the discovery, Waksman received the Nobel Prize in Medicine in 1952 . Streptomycin became very important as the first antibiotic against tuberculosis . Streptomycin is also used in agriculture to combat fire blight . No plant protection products containing this active ingredient are permitted in the EU or Switzerland . Nevertheless, in 2011 nine tons of honey were discovered in the canton of Thurgau that exceeded the tolerance level for streptomycin. In 2016, five other plant protection products were approved as alternatives to fighting fire blight.
effect
Streptomycin shows a broad spectrum of activity, with mainly gram-negative pathogens being damaged. Streptomycin binds to the 30S subunit of the prokaryotic 70S ribosomes , deforms its structure and thus inhibits the formation of the initiation complex . Accordingly, the entire translation and, as a result, bacterial multiplication are prevented.
Resistance is based on altered ribosome binding sites. Then streptomycin can even be used as a carbon source and thus promotes the growth and reproduction of resistant germs.
Because of its low therapeutic index at the same time rapid development of resistance streptomycin can only tuberculosis and few specific infections ( Streptococcus - or enterococci - endocarditis , plague , brucellosis and tularemia displayed and only as a combination therapy). It is used (as a sulfate salt) in the form of parenteral dry powder formulations.
Adverse effect
Long-term use can damage the hearing and kidneys ( ototoxicity , nephrotoxicity ).
synthesis
Numerous enzymes are involved in the biosynthesis of the compound, which, starting from glucose, initially modify the monosaccharides individually until they are linked to the disaccharide and then the trisaccharide is formed. After discharge, the precursor obtained is converted into the active form by de- phosphorylation using extracellular phosphatase. Streptomycin contains the unusual monosaccharide L-streptose .
Due to the complexity of the chemical structure, only biotechnological production comes into question economically. This is done with Streptomyces griseus strains, with a yield of more than 10 grams per liter to be expected (with a fermenter running time of 120 hours and corresponding optimizations).
Trade names
Strepto-Fatol (D), as well as a generic (D)
Individual evidence
- ↑ a b Entry on streptomycin sulfate in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ^ Entry on streptomycin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ A. Schatz, E. Bugie, S. Waksman (1944): Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. In: Proc. Soc. Exp. Biol. Med. Vol. 55, pp. 66-69.
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on streptomycin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 27, 2016.
- ↑ Antibiotics in Agriculture. In: lid.ch. October 9, 2018, accessed March 1, 2019 .