Cefalexin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
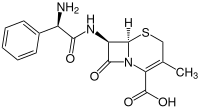
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cefalexin | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 16 H 17 N 3 O 4 S | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 347.39 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
326.8 ° C |
|||||||||||||||||||||
| pK s value |
4.5 |
|||||||||||||||||||||
| solubility |
bad in water (1.79 mg l −1 ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cefalexin is a semi-synthetic antibiotic from the class of the first generation cephalosporins .
indication
Cefalexin is mainly used for bacterial infections in the urinary and genital organs , the respiratory tract , the skin and soft tissue, the ear, nose and throat area , the bones and joints as well as the teeth .
Working principle
Cefalexin acts on bacterial cells primarily during their division by inhibiting the septum-specific synthesis of peptidoglycan . So it inhibits enzymes that cause the formation of the new cell wall during cell division. Pathogens that are multiplying are thereby damaged and ultimately killed. The effectiveness essentially depends on the period of time during which the active substance level is above the minimum inhibitory concentration (MIC) of the pathogen.
The elimination half-life with normal kidney function is about one hour. It can be prolonged in patients with kidney failure .
application
Cefalexin is taken orally (whole with liquid).
Side effects
Hypersensitivity reactions and gastrointestinal complaints (diarrhea, vomiting, nausea) are reported as possible side effects. As with all cephalosporins, there is cross-resistance with penicillins .
In addition, if there is an existing penicillin allergy, the possible cross allergy must be taken into account.
Interactions
Cefalexin can under certain circumstances falsely simulate positive results in the determination of sugar in the urine . For this reason, methods for determining urine sugar are recommended that are based , for example, on enzymatic glucose oxidase reactions. The direct Coombs test (test to determine antibodies on red blood cells) can also be false positive. Cefalexin can also interfere with the determination of ketone bodies in the urine.
Trade names
Keflex (A), Ospexin (A), Sanaxin (A) and generics (D, A)
- Veterinary medicine
Cefazid, Cephacare, CefaDog (CH), Chassot-Cefaseptin, Rilexine, Therios, Ubrolexin Suspension (combined with Kanamycin )
literature
- Michael Fresenius, Michael Heck: Repetition Intensive Care Medicine: Preparation for the examination "Special Intensive Care Medicine" . 2., completely revised and updated edition. Springer, Heidelberg 2006, ISBN 3-540-21479-8 .
Web links
- Drugs.com: information about cephalexin (English)
- Entry on Cefalexin in Flexikon , a Wiki of the DocCheck company
Individual evidence
- ↑ a b c d data sheet cefalexin from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ a b c Entry on Cefalexin in the DrugBank of the University of Alberta .
- ↑ a b ratiopharm GmbH: Product Information Cephalexin-ratiopharm 500 mg / 100 mg film-coated tablets . As of April 2018.
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 339.