Peptidoglycans
Peptidoglycan (PGN), peptidoglycans , even murein (from the Latin murus = Wall , Wall , Protection ), rare polysaccharide peptides called, are made of sugars and amino acids composite macromolecules , in the cell wall of bacteria ( murein sacculus occur). Both gram-positive and gram-negative bacteria have a peptidoglycan layer in their cell wall that gives them strength. The thickness of the shell is different, with gram-positive 20 to 80 nm, with gram-negative less than 10 nm.Exceptions are mycoplasmas and spiroplasmas , which belong to the domain of bacteria , which do not have a cell wall and therefore also no murein envelope, and the planctomycetes , which are protein-rich S-Layer cell wall.
construction

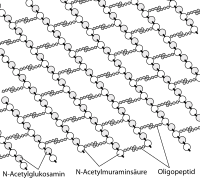
Peptidoglycans consist of strands of the two β (1 → 4) glycosidically linked sugar derivative molecules N -acetylglucosamine and N -acetylmuramic acid (see Figure 1), which form the backbone as linear chain molecules. From each N -acetylmuramic acid molecule - bound to its lactyl group - an oligopeptide chain goes to an N -acetylmuramic acid molecule of an adjacent strand (see Figure 2). The composition of both gram-positive and gram-negative bacteria is not always uniform. Most gram-positive cocci have L- lysine instead of diaminopimelic acid (DAP) . Sometimes the amino acid at position 2 is additionally hydroxylated. An important characteristic of the tetrapeptide is the presence of D- amino acids such as D -alanine or D- glutamic acid , which are enzymatically formed from the corresponding L- amino acids by racemases .
Networking
The parallel strands are cross-linked. An enzyme is required for this connection, which as a transpeptidase can link the peptide chains to one another. This transpeptidase is also known as penicillin-binding protein (PBP) because it is the target of beta-lactam antibiotics . It can have different structures and can therefore be resistant to these antibiotics .
In Escherichia coli and other gram-negative bacteria , the two tetrapeptides are directly connected (Fig. 2a). Here, the amino group of the diaminopimelic acid of one peptide is linked to the carboxy group of the terminal D -alanine of the adjacent peptide.
In Staphylococcus aureus or gram-positive bacteria , an interpeptide bridge composed of five glycine molecules (syn. Pentaglycine bridge, pentapeptide bridge) connects two tetrapeptides (Fig. 2b).
Since the connecting piece from organism to organism can contain different amino acids, this contributes to the fact that over 100 different types of peptidoglycans are known. The variance of the amino acid sequence is greater in gram-positive bacteria than in gram-negative bacteria. Only histidine , arginine or proline have not yet been detected in a connector.
As a result of the cross-linking, the murein forms a flat network that spans the surface of the bacterial cell (murein sacculus, see Fig. 3). In all cases, the sugar backbone has the same structure.
Fig. 2a: Schematic representation of the murein layer of gram-negative bacteria using the example of Escherichia coli
MurNAc = N -acetylmuramic acid;
GlcNAc = N -acetylglucosamine;
DAP = diaminopimelic acidFig. 2b: Schematic representation of the murein layer of Gram-positive bacteria using the example of Staphylococcus aureus
MurNAc = N- acetylmuramic acid;
GlcNAc = N -acetylglucosamine
Gram positive and gram negative bacteria
A bacterium is surrounded by a single murein macromolecule . Gram- negative bacteria ( behaving negatively in the Gram stain ) have a thin, single-layer murein envelope, which makes up about 5–10% of the dry mass of the bacterial envelope. In gram-positive bacteria , the thicker cell wall is made up of a multilayered murine network and teichonic acids . The proportion of murein can be up to 50% of the dry mass of the bacterial shell, which takes on Gram staining due to basic dyes .
Function, extension and meaning
The murein envelope holds the bacterial protoplasts together against the internal osmotic pressure. If the murein layer is damaged or dissolved, for example by the enzyme lysozyme , the bacterium bursts. When a bacterium grows, the murine network must therefore be expanded without creating a larger gap. Murein building blocks are synthesized in the cytoplasm and exported with the help of the lipid carrier bactoprenol (see also: Transporter (membrane protein) ). In the murein network located outside the cytoplasmic membrane , specific lytic enzymes loosen locally limited bonds in the backbone strands and in the oligopeptides and the prefabricated and exported murein building blocks are inserted by specific enzymes. The expansion of the murein therefore requires a precise interaction of different enzymes. If this interaction is disturbed, the bacterium also bursts. Some of the main antibiotics , such as B. Vancomycin and penicillin inhibit the build-up of the peptidoglycan layer.
See also
Individual evidence
- ↑ Murus bei Latinwiki.de ( Memento of the original from May 7, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed November 2, 2009.
- ^ Wissenschaft-Online-Lexika: Entry on Planctomycetales in the Lexikon der Biologie , accessed on February 15, 2012.
- ↑ a b c d Michael T. Madigan and John M. Martinko: Brock Mikrobiologie . Pearson Studies; 11th updated edition 2009; ISBN 978-3-8273-7358-8 ; P. 83f.
- ↑ K. Aktories , U. Förstermann, F. Hofmann and K. Starke : General and special pharmacology and toxicology. 10th edition. Munich, Elsevier 2009. ISBN 978-3-437-42522-6
- ↑ Wissenschaft-Online-Lexika: Entry on Murein in the Lexikon der Biologie , accessed on November 22, 2008.