D amino acids
D -amino acids are a class of amino acids wherein the functional groups - carboxy (-COOH) and amino (-NH 2 ) - α-position in D - configuration present. They are mirror image isomers of the L -amino acids.
In all known biological systems, D- amino acids are represented much less frequently than their L - isomers , which are important building blocks of life in the form of the 23 proteinogenic amino acids . For a long time it was therefore assumed that D amino acids have no biological function at all and are "unnatural". This picture has changed since the beginning of the 1990s. Today we know that D- amino acids are contained in peptide antibiotics produced by bacteria, for example , and in various plants such as rice, garlic and peas.
Some D amino acids also fulfill important physiological functions in humans . In the central nervous system in particular , these are D - serine and D - aspartic acid . D- amino acids also seem to play a role in certain diseases , such as schizophrenia . This field of research is comparatively new, and many functions of the free D- amino acids and those bound in peptides or proteins are still largely unknown or not understood.
With the help of chromatographic analysis methods, D- amino acids could be detected in a number of foods and organisms . One application here is amino acid dating to determine the age of fossils .
According to the current state of science, free D -amino acids are harmless to humans in the amounts ingested daily with food. Technically produced D- amino acids are used as building blocks for the production of (semi) synthetic antibiotics and are chemically bound components of a large number of other drugs .
Basics
Chirality
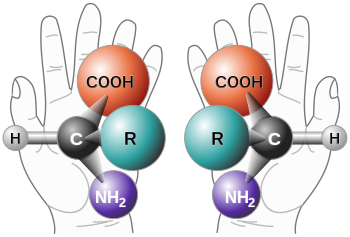
With the exception of glycine , the simplest amino acid, all proteinogenic amino acids have at least one carbon atom that carries four different atoms or groups of atoms ( substituents ). Viewed spatially, these substituents occupy the four corners of a tetrahedron . This arrangement causes an asymmetry which results in two different possibilities for the alignment of the substituents. These two forms, called enantiomers or mirror image isomers, behave like an image and a mirror image. The asymmetric carbon atom forms the so-called stereocenter . The image and mirror image of the enantiomers cannot be made to coincide. This is also the case with objects of everyday life that do not have a rotating mirror axis . The hands are an example of this. The left and right hands are like an image and a mirror image, but they cannot be brought into line. The differences between the right and left hand become particularly clear when they interact with other chiral (the Greek word for 'hand') systems. For example, when a right hand shakes a second right or left hand or tries to put on the "wrong" glove. In chiral environments, differences in the molecular enantiomers are also evident.
The German Nobel Prize laureate for chemistry Emil Fischer developed a projection method, the Fischer projection , with which the spatial structure of a chiral chemical compound can be mapped clearly in two dimensions. He chose a reference substance ( glyceraldehyde ). According to the rules of the Fischer projection, the acid group ( carboxy group ) is always drawn on top and the residue R, which distinguishes the amino acids, is always drawn below. If the amino group is on the left in this projection method ( lat. Laevus ), one speaks of an L- amino acid. The letter L is placed in front of the name of the amino acid in small caps ; for example L - serine . If the amino group in the Fischer projection is on the right-hand side (Latin: dexter = 'right'), then it is a D -amino acid. The adjectives left and right relate solely to the configuration shown according to the Fischer projection .
In terms of their physical properties , such as melting point , density , solubility in water and other solvents and isoelectric point , D - and L - amino acids are completely identical. Even in an achiral environment, i.e. in an environment in which no other chiral molecules are present, they behave the same with one exception: the two enantiomers rotate the plane of polarization of linearly polarized light under the same conditions (concentration, temperature, pH value , Solvents, etc.) the same in terms of contribution, but in different directions. If you turn the light clockwise, one speaks of clockwise or the (+) - shape. The counter-clockwise rotating form is called counterclockwise rotating or the (-) form. The sense and direction of rotation of amino acids hardly play a role in daily practice. The configuration - D - or L is much more important . The direction of rotation of an amino acid (left or right) is completely independent of the configuration of the amino acid. This is very often misrepresented in the literature. Often people speak of “levorotatory amino acids” when L -amino acids are meant. In fact, the direction of rotation and the direction of rotation are highly dependent on the external environment. For example, the amino acid L - leucine has a specific angle of rotation of + 15.1 ° (= counterclockwise) at room temperature in six molar hydrochloric acid and of −10.8 ° (= clockwise) in neutral water. In 3 molar sodium hydroxide solution , on the other hand, it is counterclockwise at 7.6 °.
A mixture of 50% D - and 50% L - amino acids is called a racemate . Racemates arise, among other things, in conventional technical syntheses of amino acids. They are optically inactive, that is, they are not able to rotate the plane of oscillation of polarized light. Compared to the pure enantiomers, racemates sometimes have different physical properties (example: melting point ), but consistently different physiological properties.
Naming conventions and nomenclature
The Fischer projection is still the preferred projection system for amino acids and saccharides . In addition, the Cahn-Ingold-Prelog convention (CIP system) is used for amino acids, which describes the absolute configuration of chiral molecules. According to the CIP system, most proteinogenic L -amino acids are ( S ) -amino acids. Their mirror images, the D -amino acids, have an ( R ) configuration almost throughout . Exceptions are L - cysteine , L - cystine and L - selenocysteine , as sulfur and selenium have a higher priority than oxygen according to the CIP nomenclature . These three L -amino acids are in the ( R ) configuration. In contrast, the three corresponding D -amino acids have the ( S ) -configuration.
In amino acid sequences , the D -amino acids in the three-letter code are preceded by a lowercase capital D (small caps).
Using the example of the heptapeptide dermorphine
H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2
In the one -letter code , D- amino acids are provided with the lower-case letter L- amino acids.
In the dermorphin example:
YaFGYPS-NH2
Natural occurrence and history of discovery
D amino acids are much rarer in nature than the isomeric L amino acids, in which the proteinogenic amino acids - together with the nucleic acids - represent the basic building blocks of life . There is a similar asymmetry in the occurrence of two types of enantiomers in the case of carbohydrates . Here the D -form, for example the D - glucose , is the “natural” configuration. It is estimated that D- glucose is 10 15 times more abundant than L- glucose on earth . There are still no reliable estimates for amino acids.
For a long time it was assumed that only the L- amino acids were selected for the formation of peptides and proteins during evolution. Since the 1980s, improved analytical methods have led to a revision of this assumption. D- amino acids have been detected in more and more living things , so that they have a significantly greater distribution and frequency than originally assumed. In the more recent literature, D- amino acids are therefore regarded as a common component of plants and foods. But also in higher living beings, including humans, D- amino acids are involved in important physiological processes, some of which are still largely not understood.
The development of life on earth required homochirality , i.e. a uniform configuration of amino acids and other building blocks of life. Self-replication cannot take place in a racemic environment . There are a number of hypotheses about the primary cause of the extreme imbalance in the frequency of the two isomeric forms of the amino acids . There is broad agreement from the point at which there was a first small imbalance in nature between the D and L configuration. From here on, the chiral amplification - a kind of self-reinforcing effect that leads in a chemical reaction to a further increase in the enantiomeric form that was previously in a slight excess - can very well explain the extreme enrichment of an enantiomeric form. However, it is completely unclear how the mirror symmetry was broken , which, with great probability, led to an initial slight excess of the L configuration in the amino acids well before the beginning of life on earth . Possible reasons for the break in mirror symmetry are, among other things, the violation of parity during β-decay ( Vester-Ulbricht hypothesis ) and the “inoculation of the primordial soup ” with extraterrestrial L- amino acid surpluses. The latter theory is supported by the fact that in the Murchison meteorite , for example, an excess of the respective L enantiomer of the non-proteinogenic amino acids 2-amino-2,3-dimethylpentanoic acid and isovaline could be detected. In the Murchison meteorite the excess of L- isovaline was about 18.5 percent and in the Orgueil meteorite about 15.2 percent. This excess was possibly generated by circularly polarized UV radiation , which - confirmed experimentally - preferentially destroys D -amino acids.
Formation of D -amino acids by racemization
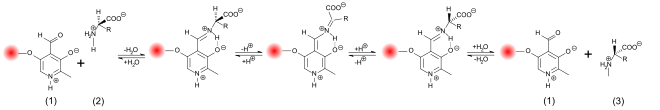
Larger amounts of D -amino acids can result from racemization from L -amino acids. The formation of an amino acid racemate, i.e. a mixture that contains 50% D - and 50% L -amino acids, is thermodynamically preferred. The enthalpy remains unchanged, but the higher “degree of disorder” leads to an increase in entropy , which means that the free enthalpy Δ G of the system decreases. The value at 25 ° C is about −1.6 kJ / mol . Higher temperatures lead to a higher release of free enthalpy, which is why the racemization is significantly accelerated. The half-life of the racemization, which is defined as the time in which the ee value falls from 100 to 50%, depends not only on the temperature, but also on the pH , the amino acid, the solvent or the humidity and the presence of catalysts . Under constant conditions, the racemization can be easily calculated in advance or, conversely, the age of the sample examined can be deduced from the degree of racemization. This process, known as amino acid dating , can be used to determine the age of fossil samples, but also of living organisms. All processes that counter the racemization of amino acids in the affected organisms end with death. Life is a struggle against entropy, and at the latest with death the processes that counteract racemization end. In some tissues with extremely low protein metabolism , this process begins after the tissue has been built up. An example of this is the collagen in the dentin of the teeth or the lens of the eye . The relatively constant temperature and pH values in the teeth make it possible to determine the age of the living organism with an accuracy of approximately ± 4 years via the degree of racemization of aspartic acid . The method is used in forensics , among other things . An example of the efficiency of this method are studies that were carried out in 1996 on the bones of Emperor Lothar von Supplinburg (1075–1137). Compared to his wife Richenza and Heinrich the proud , Lothar was found to have a much higher degree of racemization, which would correspond to an age of about 9,000 years. In contrast, the degree of racemization of the two comparison samples corresponded very well to their age of approx. 850 years. The degree of racemization of L -aspartic acid was measured in all three cases . The high degree of racemization in Lothar is due to the special circumstances of his death. He died near Breitenwang in Tyrol , about 700 km from his headquarters in Königslutter am Elm . In order to protect his corpse from decomposition before the long transport, the corpse was treated according to the “German custom” ( mos teutonicus ). The corpse of Lothar was boiled, the meat removed from the bones and the bones transferred to Königslutter. As a result of the boiling, the L -aspartic acid measured 859 years later racemized much more strongly than in the normally buried corpses of the wife and son-in-law. The cooking time could be determined to be about six hours via the degree of racemization.
In the hair of the approx. 5300 year old corpse of the man from Tisenjoch , better known as " Ötzi ", 37% of the hydroxyproline is in the D -configuration. In a 3000 year old mummy 31%, in hair from the Middle Ages (approx. 1000 years old) 19% and in fresh hair samples 4%.
The L- amino acids in the proteins can also racemize under the influence of temperature and extreme pH values when preparing food . The individual amino acids racemize at different speeds. The speed of racemization is strongly dependent on the side chain of the respective amino acid and the amino acids in its vicinity. Electron-withdrawing groups facilitate protonation of the α-C atom, which facilitates racemization. This applies to serine and aspartic acid, for example. In addition, steric effects also play a role. Asparagine and aspartic acid racemize particularly easily if the peptide sequence contains a glycine in the immediate vicinity. Then a cyclic succinimide can be formed, which thermodynamically strongly favors epimerization. At low pH values, for example in six molar hydrochloric acid, aspartic acid racemizes most strongly. Proline and glutamic acid racemize much more slowly , while isoleucine , valine , serine and threonine racemize very little under these conditions . In contrast, serine racemizes fastest in one molar sodium hydroxide solution, followed by aspartic acid, phenylalanine , glutamic acid and valine.


The base- and acid-catalyzed racemization require quite drastic reaction conditions in order to achieve a complete racemization in a few hours. In contrast, the enzyme-catalyzed racemization in biological systems takes place much faster and under very mild conditions - in the neutral pH range and at room or body temperature. The racemases catalyze the deprotonation at the α-C atom of the amino acid. The hydrogen atom is only extremely weakly acidic in this position. The acidity constant of the protonated form has a pK s value of ≈21 and the isoelectric point with ≈29 even weaker. In most racemases, the splitting off of the proton is made much easier by pyridoxal phosphate (PLP). In the active center of these enzymes, the PLP is bound to a lysine residue . The amino group of the L- amino acid binds to the aldehyde group of the PLP and thus forms a Schiff base (aldimine). As an electrophilic catalyst, the PLP draws electrons from the α-C atom of the amino acid via the aromatic ring , which deprotonates much more easily. In addition, the remaining anion is stabilized mesomer . Reprotonation and addition of water then releases the racemized amino acid as a reaction product through hydrolysis of the Schiff base.
There are also PLP-independent racemases, in whose active center the thiol groups of two cysteines catalyze the protonation. In a two-base mechanism, a deprotonated thiolate (RS - ) initially takes up the proton of the α-C atom as a base. The thiol group of the second cysteine is then responsible for reprotonation. These enzyme-catalyzed racemization processes produce the vast majority of D -amino acids in organisms.
Peptide antibiotics and other peptide drugs of natural origin

A large number of peptide antibiotics are made up of D- amino acids. Peptide antibiotics are natural products that are produced by prokaryotes using nonribosomal peptide synthesis . The pharmacologically very important group of penicillins contains D - penicillamine , a non-proteinogenic α-amino acid , as an elementary component . The polymyxins (in Polymyxin B1 D -phenylalanine) and actinomycins ( D -valine) are also made up of D -amino acids. The bacitracin formed by bacteria of the species Bacillus subtilis consists of D -aspartic acid, -glutamic acid, -ornithine and -phenylalanine. The by Streptomyces fulvissimus produced valinomycin contains D -valine and that of Bacillus circulans formed circulin A ( D -leucine). Also Fungisporin ( D -phenylalanine and D -valine), gramicidin and tyrocidin (both D -phenylalanine), malformin C ( D -leucine and D -cysteine), Mycobacillin ( D -aspartic acid and D -glutamic acid) are peptide antibiotics with D -Amino acids.
The immunosuppressant ciclosporin excreted by tubular fungi such as Tolypocladium inflatum contains D -alanine. In isopenicillin N is D -valine included.
The cycloserine , which is used to treat tuberculosis and has a relatively simple chemical structure , is produced from D- serine by streptomycetes such as Streptomyces garyphalus ,
D- amino acids and D- amino acids-containing peptides

For a long time it was assumed that only one amino acid enantiomer, namely the L form, predominates in nature . D- amino acids were regarded as “laboratory artifacts” (system-dependent errors) and classified as “unnatural isomers” until the 1960s. The term "unnatural amino acids" can still be found today for the D- amino acids .

D- amino acid oxidases - enzymes without a substrate?
In 1933, the German physician, chemist and later Nobel laureate for physiology or medicine Hans Adolf Krebs discovered the enzyme D - amino acid oxidase and described it in detail two years later. Krebs found that the "non-naturally occurring" D- amino acids, in the presence of pureed fresh pork kidney or liver , are deaminated significantly faster than their "natural" L -isomers . Through targeted inhibition , for example with 1-octanol , he was able to deactivate the L -amino acid oxidase contained in the puree and thus achieve that only the D -amino acids were selectively deaminated. Concluded from cancer that used in the organs, or their extracts, two amino acid oxidases were that L - and D , -Aminosäureoxidasen which each selectively L - or D -amino acids as a substrate have. Krebs was surprised that there is an enzyme that only has “non-natural substances” as a substrate. However, he pointed out that Felix Ehrlich in 1914, Edmund Oskar von Lippmann in 1884 and Sigmund Fraenkel in 1923/24 described the occasional occurrence of D -amino acids in nature. D -alanine, isolated from boletus mushrooms ( Boletus edulis ) by E. Winterstein and colleagues in 1913 , was one of these early records.
D amino acids in plants
D- amino acids can be detected in plants both in free form and in peptide-bound form. They are often in the form of N - malonyl - or N - acetyl - derivatives contained in the plants. For example, 40% of the alanine in the root of the sunflower ( Helianthus annuus ) is in the D configuration. D -alanine and the dipeptide D -Ala- D -Ala are found in various grasses; so also in rice ( Oryza australiensis ). Approx. 10% of the serine in rice is present as the D enantiomer. It is generated by the plant itself with the help of a serine racemase. The corresponding gene for this enzyme is in Oryza sativa ssp. Japonica cv. Nipponbare on chromosome 4. D- amino acids were found in lower concentrations in a large number of plants that are used as food. These include, for example, peas ( Pisum sativum ), garlic, various types of cabbage and fruit. The function of the free and peptide D -amino acids in plants is still largely unclear.

Bacteria and D amino acids
Prior to detection of free D microbial origin were in a series of compounds-amino acids D -amino acids identified. For example , benzylpenicillin , which was formed in mold cultures and was the first penicillin to be discovered by Alexander Fleming in 1928, contains D -penicillamine (= 3-mercapto- D- valine) as an essential element . The biochemist Esmond E. Snell noticed in 1943 in experiments with cultures of Streptococcus faecalis and Lactobacillus casei that the pyridoxine (vitamin B 6 ) necessary for the growth of these bacterial strains can be completely replaced by D -alanine as a nutrient. He also found that D -alanine was significantly more potent than L -alanine. When it was then possible to detect large amounts of D -alanine in peptidoglycans - these are the biopolymers that give the cell wall of bacteria its strength - it was clear what the cells need this "unnatural" amino acid for. The incorporation of D -alanine, and especially of D -glutamate, prevents the enzymatic breakdown of the peptidoglycans by peptidases . Interestingly, it is precisely this “protective wall” made of D- amino acids that is the point of attack for β-lactam antibiotics such as penicillin. These antibiotics inhibit the enzyme D-alanine transpeptidase , which is only present in bacteria and catalyzes the cross-linking of the peptidoglycans, especially via the D -alanine . In 1951, Irwin Clyde Gunsalus and Willis A. Wood isolated alanine racemase from Streptococcus faecalis , an enzyme that catalyzes the racemization of natural L -alanine into isomeric D -alanine. The alr gene, which codes for alanine racemase, is present in all bacteria. The D -alanine formed with the help of alanine racemase is essential for the synthesis of peptidoglycans in almost all bacteria. In addition to D -alanine and D -glutamic acid, certain strains of enterococci also contain D -serine in the cell wall. The D -Serine forms a D -Ala- D -Ser dipeptide with D -alanine at the C-terminus , which is responsible for the resistance of these bacterial strains to glycopeptide antibiotics such as vancomycin.
D amino acids in sponges
So-called polytheonamides could be detected in sponges . These are peptide toxins whose amino acids alternate between the D and L forms . They are apparently synthesized ribosomally as L -peptides and then post-translationally every second amino acid is epimerized. This is done with the help of a few enzymes whose genes, obviously derived from bacteria, have entered the sponges via horizontal gene transfer .
D amino acids in multicellular cells
Dankwart Ackermann and M. Mohr were able to detect D - ornithine in the liver of the dogfish ( Acanthias vulgaris ) in 1937 . The D- amino acid oxidase discovered by Krebs was detected in all mammals in the following years . H. Blaschko and Joyce Hawkins first found them in invertebrates in 1951 . However, the function of this enzyme in the various organisms remained unclear. Towards the end of the 1960s, it was speculated that the enzyme was used in the digestive tract to break down cell wall components of gram-positive bacteria that contain large amounts of D -amino acids. The theory that D -amino acid oxidase only serves to break down externally supplied (exogenous) D -amino acids existed until the early 1990s.
In the hemolymph of Wanzenart Oncopeltus fasciatus was Auclair and Patton first time to a 1950 multicellular D detected alanine. They used two-dimensional paper chromatography as the analytical method . After eluting, they sprayed the dried chromatograms with D -amino acid oxidase, which deaminated only the D -alanine to a ketocarboxylic acid, which could easily be detected with phenylhydrazine . The reason for the presence of D -alanine was suspected to be the microbial flora, ingestion through food and spontaneous racemization through aging .
The biosynthesis of D -serine was demonstrated in 1965 by a group led by John J. Corrigan at Tufts University School of Medicine in Massachusetts . The silk moths fed with radioactively labeled D - glucose produced both L- and D- serine. Later, D -amino acids in other insects detected and mammals.
1962 isolated an Italian research group led by Vittorio Erspamer in the South American frog of Physalaemus fuscomaculatus the tachykinin physalaemin . This polypeptide is made up of twelve amino acids and, viewed from the N-terminus, begins with D- proline. In the one-letter code, the sequence is pEADPNKFYGLM-NH2. It was the first natural peptide to be discovered with a D -amino acid that is not of microbiological origin. But even three years later, for example, the American biochemist Alton Meister wrote in his standard work Biochemistry of the amino acids that “there is currently no conclusive evidence for the occurrence of D- amino acids in the proteins of plants and animals” . At first hardly any notice was taken of Erspamer's discovery. It was only 19 years later when the same working group isolated dermorphin in the warty macro frog ( Phyllomedusa sauvagii ), which is also native to South America , that the scope of the discovery was slowly recognized. Viewed from the N terminus, the dermorphin, which is composed of seven amino acids, has a D -alanine at position 2 . The D configuration of alanine is essential for pharmacological activity. Dermorphin binds to the µ 1 receptor and is much more selective and potent than the body's own endorphins ( dynorphins and enkephalins ) and the pharmacologically widespread plant morphine . The discovery contradicted some paradigms, so that Erspamer had considerable difficulties in finding a journal that published the results of his working group. One of these paradigms is that during protein biosynthesis , the DNA of an organism only codes for the 20 canonical amino acids , which are exclusively in the L configuration . There is no gene coding for D- amino acids. This contradiction was resolved over ten years later: a stereoselective post-translational modification catalyzed by epimerases is responsible for the occurrence of D -amino acids in eukaryotic peptides. This means that after translation, under the influence of a special, endogenous enzyme, the configuration of a certain L- amino acid is changed.
D amino acids in mammals
A biological function of the D -amino acids in mammals was ruled out until 1992. The improvement of analytical measurement methods such as gas and high-performance liquid chromatography (GC or HPLC) made it possible from the 1980s to cleanly separate D- amino acids from their L- mirror images and to detect them in even the smallest quantities. In 1992 Atsushi Hashimoto and colleagues found relatively large amounts of free D -serine in the brain of rats . They found a concentration of about 0.27 µmol / g brain mass. They determined the L -serine content to be 0.89 µmol / g brain mass, which resulted in a D- to- L ratio of 0.23. It was already known before that D -serine supplied externally (exogenously) is a potent selective allosteric agonist at the NMDA receptor ( N -methyl- D -aspartate). The source of the comparatively high concentrations of D -serine, which was subsequently also detected in the brains of other mammals, including humans, initially remained unclear. Speculations, such as the targeted uptake of racemized L- serine from food and transport across the blood-brain barrier into the brain, ended in 1999 with the discovery of the enzyme serine racemase in the brain of rats by Herman Wolosker and colleagues. Serine racemase catalyzes the racemization of serine. Amino acid racemases were previously only known in bacteria and some insects. The enzyme was detected in glial cells that have comparatively high concentrations of D -serine. With the detection of the serine racemase it could be shown that this archaic D -amino acid metabolism is also conserved in mammals and - as was to be shown later - there exercises an important function in the neurotransmission . The dogma that D- amino acids have no special functions in eukaryotes had to be abandoned. Today we know that D- serine plays an important role in numerous processes of the central nervous system , such as learning processes and memory function , but also in mental illnesses , neuropathies and neurodegenerative diseases .
Physiological importance
Free D amino acids

Until the end of the 1990s, it was assumed that D -amino acids had no physiological function in vertebrates. Research into the function of these two extraordinary amino acids began with the detection of large amounts of D -serine and D -aspartic acid in the brain of mammals. Research into the physiological effects of D -amino acids is a comparatively young discipline with many unanswered questions.
D -Serin
In addition to glial cells , D -Serine isalso found in nerve cells (neurons). It arises from L -serine under the catalytic influence of the enzyme serine racemase ( EC 5.1.1.18) derived from these cells expressed is. D- amino acid oxidase (EC 1.4.3.3) catalyzes thedegradation. The concentration of D -serine in the brain is determined by these two build-up and breakdown processes. D -Serine acts as a co-agonist on the NMDA receptor , whose “natural” ligand is the amino acid glycine. This receptor is of great importance for a number of physiological, but also pathological processes. D -Serine increases the activity of the NMDA receptor. It is therefore also called a ' neuromodulator '. Overexpression of D -amino acid oxidase, which leads to increased degradation of D -serine, consequently reduces the activity at the NMDA receptor. An underactive NMDA receptor is mainly associated with schizophrenia . Even small amounts of NMDA receptor antagonists cantrigger symptoms such as cognitive and physiological disorders that correspond to those of schizophreniain healthy test persons .
In 2002 a large international working group found that the newly discovered G72 gene ( DAOA gene, D-amino acid oxidase activator ) is closely related to schizophrenia. The gene product of G72 activates D- amino acid oxidase, which decreases the concentration of D -serine in the brain. They found only a weak correlation between the activity of D- amino acid oxidase and the occurrence of schizophrenia. The combination of D- amino acid oxidase and G72 activator was strongly mutually supportive ( synergistic ). The authors concluded that ultimately the concentration of free D -serine plays an essential role in schizophrenia. Other studies have also shown a genetic link between D- amino acid oxidase and schizophrenia. The results of working groups, which were able to prove that the concentration of D -serine in the blood serum and in the cerebrospinal fluid of schizophrenia patients, compared to a group of healthy test persons, are significantly reduced in line with these findings . In addition, an increased expression of D- amino acid oxidase was found in the brains of deceased schizophrenic patients. The addition of D -serine in the treatment of patients with schizophrenia showed promising results in clinical trials. A meta-analysis of 18 clinical studies found a reduction in symptoms of schizophrenia. The improvement was only moderate, however.
The knowledge of the function of D- amino acid oxidase and D- amino acid oxidase has led to the development of various inhibitors of D- amino acid oxidase, which are potential drugs for the treatment of schizophrenia. The D- amino acid oxidase inhibitors are still in a very early development phase, so that no medicinal product with this active principle has yet been approved (as of 2012).
An excessive concentration of this amino acid in glial cells and the associated excitotoxicity is being investigated as a possible cause of amyotrophic lateral sclerosis , a degenerative disease of the nervous system.
D -aspartic acid
Free D -aspartic acid was detected for the first time in 1986 by a working group headed by the American David S. Dunlop in significant amounts in the brains of rodents and in human blood. They found the highest concentrations of D -aspartate in the cerebral hemisphere of newborn rats at 164 nmol / g . This corresponded to 8.4% of the total amount of aspartic acid. This concentration value exceeds that of many essential L -amino acids in the brain. In addition to the brain, comparatively high amounts of D- aspartate were found in the pineal gland , the pituitary gland , the adrenal glands and the testes . Analogously to D -serine, D -aspartate is formed in the organism by enzymatic racemization of L -aspartate, in this case by D -aspartate racemase (EC 5.1.1.13), and the degradation takes place via D-aspartate oxidase (EC 1.4.3.1). The concentration of D -aspartate decreases drastically with increasing age of the organism. High activities of D -aspartate racemase are found in the organs in which high concentrations of D -aspartic acid can also be detected. The activity is highest in the pituitary gland. Deactivation of the aspartate racemase, for example by retroviruses , which specifically cause a loss of function in the ribonucleic acid (RNA), which is complementary to the aspartate racemase , leads to a significant decrease in the concentration of D -aspartate. As a result, the dendritic development is massively disturbed, which in turn leads to pronounced damage to neurogenesis in the hippocampus . Based on these test results , it is assumed that D- aspartate is an important regulator of neuronal development. The exact physiological effects of D -aspartic acid are still largely unclear. The research area is very new. For example, aspartate racemase was only cloned in mammals in 2010 .
D- amino acid containing peptides
As an organism ages, the increased racemization, especially of aspartic acid, leads to an increasing loss of homochirality. Oxidative stress and UV radiation can accelerate this loss. The racemization (engl. Of aspartic acid aspartic acid racemization ) proceeds because of the formation of a succinimide intermediate can that only a low activation energy required particularly easily. This non-enzymatic in vivo racemization of proteins is an autonomous process of aging that primarily affects long-lived proteins such as collagen in dentine or crystalline in the lens of the eye. For example, 0.14% of the aspartic acid in the lenses of the eye racemizes every year of life. In a 30-year-old, an average of 4.2% of the aspartic acid in the crystalline of the eye lenses is racemized. In addition, however, other functional proteins, such as enzymes or messenger substances, are also affected by racemization. Peptides that contain D amino acids are significantly more stable to enzymatic degradation by proteases than peptides whose amino acids are only in the L configuration. In many cases, racemization in an endogenous protein leads to physiological problems. In proteins, racemization causes a loss of function and an accumulation of the protein in a wide variety of tissues, which the organism can no longer break down. An increase in racemization can be observed in some clinical pictures. In atherosclerosis , emphysema , presbyopia , cataracts and signs of degeneration of the cartilage and brain, the racemization of aspartic acid is seen as a relevant pathological factor.
In 1988, an increased degree of racemization was found for the first time in the β-amyloid of the senile plaques from the brain of deceased patients with Alzheimer's disease . Above all D -aspartate and D -serine could be detected. It was later recognized that racemization of aspartic acid in position 23 leads to accelerated peptide aggregation, which is seen as an essential element in the pathogenesis of Alzheimer's disease. In contrast to the racemization in position 23, the racemization in position 7 leads to reduced peptide aggregation. An important role in the development of Alzheimer's disease is ascribed to the racemization processes of β-amyloid, which are probably caused by protein aging and which proceed similar to those in dentin. Racemization accelerates peptide aggregation and makes enzymatic degradation by proteases more difficult.
properties
Chemical and physical properties
In an achiral environment, D and L amino acids are completely identical in their chemical and physical properties, with the exception of the direction of rotation of polarized light. Significant differences can be found in a chiral environment. This is especially true with biochemical processes that are inherently chiral. A practical example of this is the difference in taste between the amino acid enantiomers. The G-protein-coupled taste receptors made up of L- amino acids are a chiral environment with which enantiomers interact differently. The taste of most L amino acids is described as ' bitter ', while that of D amino acids is usually described as 'sweet'. An extreme example is D - tryptophan ; by far the sweetest amino acid has 37 times the sweetness of sucrose. L -Tryptophan, on the other hand, is together with L -Tyrosine the most bitter amino acid. The interactions with other receptors or enzymes in biochemical processes can be correspondingly different. This also applies in particular to peptides and proteins that contain one or more D -amino acids.
The incorporation of a D or the epimerization of an L amino acid in a protein causes, from a stereochemical point of view, the formation of a diastereomer that gives the entire protein completely new chemical and physical properties. Biochemically, this intervention in the primary structure has considerable effects on the secondary , tertiary and quaternary structure of the peptide derived from it . The biochemical effect is greatly changed. In the two extreme cases, it can either be completely lost ( loss of function ) or completely new, for example toxic effects ( gain of function ). In a peptide otherwise composed of L -amino acids, D -amino acids prevent an α-helix from being formed. They are 'helix breaking'. Only proteins that are completely made up of D or L amino acids can - if helix-forming amino acids such as valine, glutamine , isoleucine, alanine, methionine , leucine, glutamic acid or tryptophan are present - form a helical structure that are mirror images of each other. This is not possible with mixed peptides.
toxicology
D -isomers of proteinogenic amino acids
In studies in which the extensive oral intake of amino acids - for example in the form of food supplements - was investigated, all amino acids in the “natural” L configuration , with the exception of serine and aspartic acid, showed more toxic effects than the corresponding D enantiomer. D amino acids are a natural component of a wide variety of foods. There they arise mainly through racemization processes from the “natural” L- amino acids. Food that has undergone a fermentation process, such as dairy products , has increased levels of D- amino acids. Emmentaler contains around 0.7 g / kg of D amino acids. Even in the starting product, cow's milk , around 1.5% of all amino acids are in the D configuration.
It is estimated that about a third of the D amino acids ingested through food are of microbial origin . In order to be able to use the amino acids contained in food and linked in proteins for the organism, the proteins have to be broken down into their individual components, the free amino acids, during digestion. If there are D -amino acids in a protein , the accessibility of the protein to proteolytic enzymes can be considerably restricted. The enzymes of the human digestive system cannot break bonds between D - and L - amino acids. The breakdown into individual amino acids, di- or tripeptides, which is necessary to be able to be absorbed by the organism through the intestinal mucous membranes , is made more difficult. Larger peptide fragments cannot be used and are excreted with the faeces . The bioavailability , and thus also the nutritional value, is then greatly reduced. Di- or tripeptides which contain D -amino acids can - like free D -amino acids - be absorbed via peptide transporters . A large part of the D amino acids absorbed in this way is excreted via the kidneys. Depending on the food supply and the respective D -amino acid, some of the D -amino acids are converted into L -amino acids by transamination and thus made accessible for protein biosynthesis .
The incorporation of “unnatural” D- amino acids into the cell wall of bacteria makes them resistant to proteases. This protease stability is also of great importance for humans, after all there are several hundred grams of intestinal bacteria in an adult's intestine , which, together with a large number of proteases, are essential for digestion.
Most of the D- amino acids in food are produced during preparation. High temperatures and strongly acidic or basic conditions lead to (partial) racemization. For example, about 14% of the aspartic acid in potato chips is in the D form. In coffee whiteners it is 17% and in strips of bacon 13%. Free L -amino acids racemize about ten times more slowly than protein-bound ones. The degree of racemization is also highly dependent on the amino acid itself. Serine tends to racemize particularly easily due to the hydroxyl group . The drastic conditions required in the production of gelatine - either acidic or basic digestion at elevated temperatures - lead to strong racemization, especially of aspartic acid, in the collagen of the gelatine. The proportion of D -aspartate in the total aspartate can be slightly above 30% in commercially available gelatine.

D- amino acids are not incorporated into proteins or peptides or other (macro) molecules of the metabolism when they are taken up by the mammalian organism. An accumulation in the body tissue in unchanged form cannot be observed. Through food or by infusion recorded D -amino acids are partly on the urine excreted and partly via the existing in liver and kidney enzyme D -Aminosäureoxidase by deamination in the "normal" metabolic products, the keto carboxylic acids , oxidized . With regard to the toxicity of infused D -amino acids, there is, more or less involuntarily, many years of experience which suggest that D -amino acids are not harmful to health. The basis of this statement is the good tolerance of parenteral nutrition ("artificial nutrition"), which for many years consisted of high-dose amino acid racemates. These infusion solutions were made from proteins by means of acid hydrolysis - which inevitably leads to racemization. Racemic methionine ( DL -methionine) is a component of many feed in the livestock industry . In dairy cows it has been shown that over 75% of D- methionine is transformed into L- methionine and thus becomes bioavailable.
Independent of these empirical values, test results can be seen on the rat animal model. High doses (in the range of 0.8 g / kg body weight) of D -serine lead to acute tubular necrosis in these model organisms , which is reversible after discontinuation of D -serine administration. The kidney function is fully regenerated after about six days. The pathological changes are largely similar to those of kidney damage caused by lysinoalanine . Why D -serine is toxic to the kidneys in these high concentrations has not yet been clarified with certainty. The D -serine may reduce the concentration of renal glutathione , which is supposed to protect the proximal tubular cells from the harmful effects of reactive oxygen species (ROS). The enzymatic degradation of D -serine by D -amino acid oxidase produces hydrogen peroxide as a by-product , which significantly reduces the intracellular supply of glutathione.
In December 1989, a message from three doctors from Vienna, published in the prestigious journal The Lancet , caused a sensation . They had found large amounts of D - proline in milk that they heated up in a microwave oven , which was apparently produced by the racemization of L- proline . They also attributed neurotoxic , nephro- and hepatotoxic properties to D -proline . The publication was a letter to the editors and not a peer-reviewed publication or even a controlled study. The authors also did not name the test conditions under which this degree of racemization was achieved. Independently of this, the report was published in the daily and weekly press with dramatic formulations and warnings against the use of microwave devices. In August 1990 the Federal Health Office clarified the facts, but this had little public effect. Other scientists indicated that D- proline is a normal part of daily food that is quickly broken down and excreted after ingestion. Nevertheless, in August 1991, for example, a magazine appeared with the headline “Microwaves poison nerves, liver and kidneys” . Similar claims can still be found on relevant websites today.
Attempts by other working groups to reproduce the results of the Viennese doctors initially failed. Even if milk was boiled on the stove for 30 minutes, no increase in D- proline could be measured. The test conditions were published two years later. The authors of the Lancet-Letter had heated the milk in a closed pressure vessel for 10 minutes to 174 to 176 ° C - a temperature range that cannot be reached in normal household vessels for heating milk. In their statement on the neurotoxicity of D- proline, the authors of the Lancet Letter referred to experiments from 1978 in which chicks were injected with the substance intraventricularly , i.e. directly into a cerebral ventricle . Subsequent studies on the toxicity of D- proline in rats showed that the compound is harmless even in high concentrations.
A real danger when heating milk with a microwave device - especially for small children - is the uneven heating of the bottle contents, which often leads to clinically relevant burns.
D -isomers of non-proteinogenic amino acids
No general statements can be made about the toxicity of the D isomers of non-proteinogenic amino acids. It is very individual from amino acid to amino acid. Interestingly, some compounds containing D amino acids are significantly less toxic than their L isomers. Examples are cycloserine and penicillamine . For example, which is LD 50 value for the oral administration of the racemate of D - and L -Penicillamine the model organism rat at 365 mg / kg. For the pure D- penicillamine, however, there are no signs of toxicity even at a dose of 1200 mg / kg.
D peptides
General statements about the toxicological properties of D -peptides are not possible. The sensitivity to proteases is significantly lower and the immunogenic potential is significantly lower than that of the corresponding L -peptides.
analysis
Classic procedures
The optical angle of rotation of an amino acid solution can be determined with a polarimeter , from which the content of D and L enantiomers can be calculated. However, standardized conditions (especially concentration, temperature and solvent) are necessary for this. In addition, the method is only suitable for individual amino acids and not for mixtures of different amino acids. In the 1960s to 1980s, ion exchange chromatography was also used to separate derivatized amino acids. The amino acids to be analyzed were converted into diastereomeric dipeptides with L- amino acids before the separation . Enzymatic methods that are based on the reaction with specific enzymes such as L - and D - amino acid oxidase belong to the classic methods of determining the enantiomers of amino acids. Capillary electrophoresis , among other things, is also suitable as a non-chromatographic method for the analysis of D -amino acids.
Chromatographic Process
Quantitative analyzes, even of complex mixtures of amino acids, can be carried out with the help of chromatographic methods . The individual components of the mixture are first separated on a stationary phase and then measured with a detector. UV or mass spectrometers are used as detectors , and flame ionization detectors are also used in gas chromatography . Two different strategies are used to separate the starting mixture at the stationary phase. In the simplest case, the two enantiomers are separated on a chiral stationary phase with which the two isomers interact to different degrees and thus elute at different speeds . Separation is only possible on an achiral stationary phase if the enantiomers are converted into diastereomers. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) have established themselves as analytical methods. The enantiomeric purity of D -amino acids can also be analyzed by thin layer chromatography .
Only the development of special chromatographic methods made it possible to detect and quantify D- amino acids in the organs of higher organisms.
Gas chromatography
Amino acids cannot be evaporated without decomposition. For separation and analysis in gas chromatography, they have to be converted into compounds that can be vaporized without decomposition. For this purpose, the amino acids are usually subjected to a two-stage derivatization process. For example, the carboxy group can be esterified with ethanol in the first step and then, in a second step, the amino group can be converted with trifluoroacetic anhydride to give the trifluoroacetyl derivative (TFA). The N -TFA / O -ethyl derivative of the amino acid formed in the process can be evaporated without decomposition in the gas chromatograph and separated on a chiral stationary phase. Derivatization with chiral reagents entails the increased risk of racemization and that the reaction partners have different reaction kinetics. Both can falsify the measurement result.
High performance liquid chromatography
In HPLC, compared to gas chromatography, derivatization with chiral reagents and the use of non-chiral stationary phases, for example RP-18 , have prevailed. For example, L - N -acetylcysteine is used together with phthaldialdehyde for derivatization . The resulting pair of diastereomers (D - L and L - L) has different chemical and physical properties, which means that it can be separated on a conventional column and then detected.
synthesis
Most proteinogenic L amino acids are produced by fermentation . This microbiological process is not suitable for D -amino acids. Various production processes have been developed to meet the increasing demand for D -amino acids.
The classic chemical syntheses, such as the Strecker synthesis , always yield the racemates of the amino acids. From these mixtures, the individual amino acids either are separated off-consuming (can resolution ) or adding the L -amino acid enzymatically using L - Aminosäuredesaminasen to the ketocarboxylic acid to which can be comparatively easily separated.
The D- amino acid synthesis via substituted hydantoins is more elegant . Hydantoins can be produced on an industrial scale according to the Bucherer-Bergs reaction (also called Bucherer-Bergs hydantoin synthesis ) from aldehydes, potassium cyanide and ammonium carbonate . The amino acid formed is determined by the choice of aldehyde used. The hydantoin produced in this way can be converted into D -amino acid in the so-called hydantoinase process . This multi-enzyme process was developed by Degussa (now Evonik Degussa ) and consists of three reaction steps. First, the racemic hydantoin derivative is under the catalytic influence of D - hydantoinase to N carbamoyl- D hydrolyzed amino acid. In the second step, the N -carbamoyl- D- amino acid is further hydrolyzed to the enantiomerically pure amino acid with the aid of a D- carbamoylase . In the third step, the enantiomer of the hydantoin derivative that has not been converted during the synthesis is chemically or enzymatically racemized. Chemical racemization takes place at pH values> 8 and can be significantly accelerated by adding a racemase. Compared to other processes, the hydantoinase process produces enantiomerically pure amino acids with theoretical yields of up to 100% starting from the racemate.
use
The worldwide demand for D -amino acids has increased continuously over the last few years. For 2017, a market size of around 3.7 billion US dollars is forecast.
D- amino acids are found as important building blocks, for example, in sweeteners , insecticides , cosmetics and, above all, in a large number of peptide drugs that are a major growth driver for market development.
Several thousand tons of D -4-hydroxyphenylglycine and D -phenylglycine are required for the synthesis of penicillins (for example amoxicillin ) and cephalosporins (for example cefaclor ) every year .
D- amino acids not only increase the stability in the cell walls of bacteria against proteolytic degradation, the targeted incorporation into drugs also increases their stability, especially when taken orally . The change in the arrangement of the functional groups ( conformation ) also offers a further degree of freedom in the design of the molecular structure in the molecular structure, which can lead to improved drug properties. The gonadorelin inhibitor cetrorelix , a GnRH analog used in reproductive medicine , consists, for example, of ten amino acids, five of which are in the D configuration. Cetrorelix is built up fully synthetically from the individual amino acids. Other GnRH analogs such as leuprorelin , buserelin , degarelix , histrelin , nafarelin or abarelix also contain at least one D- amino acid.
For the treatment of erectile dysfunction used tadalafil , better known under the brand name Cialis is, in the synthesis of D constructed tryptophan. The antidiabetic nateglinide , from the Glinde group , is made from D- phenylalanine and cis -4-isopropyl-cyclohexane-carboxylic acid. Phenylalanine has been used as an antidepressant since the 1970s . The inexpensive racemate is used as a drug. A significant part of the antidepressant and analgesic effect comes from D- phenylalanine, which - in comparison to L -phenylalanine - is not metabolized into mood- enhancing L- tyrosine, L- DOPA or noradrenaline , but primarily inhibits the enzyme enkephalinase . By blocking the enkephalinase, the blood level of enkephalins in the blood is increased, which causes the analgesic effect that can also be observed . In the further course of the process, the D- phenylalanine is then mainly metabolized to phenylethylamine .
The insecticide fluvalinate from the group of pyrethroids , which is approved, among other things, for combating the varroa mite is made from D- valine.
D -alanine is a component of the sweetener alitame .
further reading
- Ryuichi Konno, Hans Brückner, Antimo D'Aniello, George Fisher, Noriko Fujii, Hiroshi Homma: D-amino acids: a new frontier in amino acids and protein research - practical methods and protocols. Nova Science Publishers, 2007, ISBN 1-60021-075-9 , 629 pp.
- Loredano Pollegioni, Stefano Servi (Ed.): Unnatural Amino Acids. Humana Press, 2011, ISBN 1-61779-330-2 , 409 pp.
- Gyula Pályi, Luciano Caglioti, Claudia Zucchi (eds.): Advances in BioChirality. Elsevier, 1999, ISBN 0-08-043404-5 ( limited preview in Google Book Search).
Web links
- The D and L forms of the amino acids
- Hanka Symmank: Functional and structural characterization of bacterial peptide synthetases. Department of Biology, Chemistry, and Pharmacy, Free University of Berlin, May 2002
Individual evidence
- ↑ a b Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Textbook of food chemistry. 5th edition, Springer Verlag, 2001. ISBN 3-540-41096-1 ( limited preview in the Google book search).
- ↑ a b c d Uwe Meierhenrich : Amino Acids and the Asymmetry of Life: Caught in the Act of Formation. Springer, 2008, ISBN 3-540-76885-8 , pp. 53–54 ( limited preview in Google book search).
- ↑ VS Lamzin, Z. Dauter, KS Wilson: How nature deals with stereoisomers. In: Current opinion in structural biology. Volume 5, Number 6, December 1995, pp. 830-836, PMID 8749373 . (Review).
- ^ SA Fuchs, R. Berger u. a .: D-amino acids in the central nervous system in health and disease. In: Molecular Genetics and Metabolism. Volume 85, Number 3, July 2005, pp. 168-180, doi: 10.1016 / j.ymgme.2005.03.003 . PMID 15979028 . (Review).
- ↑ GF Joyce, GM Visser u. a .: Chiral selection in poly (C) -directed synthesis of oligo (G). In: Nature. Volume 310, Number 5978, 1984 Aug 16-22, pp. 602-604, PMID 6462250 .
- ↑ VV Avetisov, VI Goldanskii: Homochirality and stereospecific activity: evolutionary aspects. In: Bio Systems. Volume 25, Number 3, 1991, pp. 141-149, PMID 1912384 .
- ↑ N. Fujii, T. Saito: Homochirality and life. In: Chemical record. Volume 4, number 5, 2004, pp. 267-278, doi: 10.1002 / tcr.20020 . PMID 15543607 . (Review).
- ^ WA Bonner: Experimental evidence for beta-decay as a source of chirality by enantiomer analysis. In: Origins of life. Volume 14, Numbers 1-4, 1984, pp. 383-390, PMID 11536584 . (Review).
- ^ WA Bonner: Parity violation and the evolution of biomolecular homochirality. In: Chirality. Volume 12, Number 3, March 2000, pp. 114-126, doi : 10.1002 / (SICI) 1520-636X (2000) 12: 3 <114 :: AID-CHIR3> 3.0.CO; 2-N . PMID 10689289 . (Review).
- ↑ JR Cronin, S. Pizzarello: Enantiomeric excesses in meteoritic amino acids. In: Science. Volume 275, Number 5302, February 1997, pp. 951-955, PMID 9020072 .
- ↑ S. Pizzarello, M. Zolensky, KA Turk: Nonracemic isovaline in the Murchison meteorite: chiral distribution and mineral association. In: Geochimica et Cosmochimica Acta. Volume 67, Number 8, 2003, pp. 1589-1595. doi: 10.1016 / S0016-7037 (02) 01283-8 .
- ↑ P. Schmitt-Kopplin , Z. Gabelica u. a .: High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. In: PNAS. Volume 107, Number 7, February 2010, pp. 2763-2768, doi: 10.1073 / pnas.0912157107 . PMID 20160129 . PMC 2840304 (free full text).
- ↑ DP Glavin, JP Dworkin: Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. In: PNAS. Volume 106, Number 14, April 2009, pp. 5487-5492, doi: 10.1073 / pnas.0811618106 . PMID 19289826 . PMC 2667035 (free full text).
- ↑ PW Lucas, JH Hough et al. a .: UV circular polarization in star formation regions: the origin of homochirality? In: Origins of life and evolution of the biosphere. Volume 35, Number 1, February 2005, pp. 29-60, PMID 15889649 .
- ^ A b T. Carell : ( Page no longer available , search in web archives: Lecture Stereochemistry. ) Chapter 9: Racemizations LMU Munich, p. 150.
- ↑ Elizabeth R. Neswald: Thermodynamics as a cultural battleground: on the history of fascination with entropy, 1850-1915. Rombach, 2003, ISBN 3-7930-9448-0 , p. 335.
- ↑ AS Kekulé: Oh, how good that nobody knows ... In: Tagesspiegel. 12 January 2011.
- ↑ T. Ogino, H. Ogino: Application to forensic odontology of aspartic acid racemization in unerupted and supernumerary teeth. In: Journal of dental research. Volume 67, Number 10, October 1988, pp. 1319-1322, PMID 3170888 .
- ↑ T. Ogino, H. Ogino, B. Nagy: Application of aspartic acid racemization to forensic odontology: post mortem designation of age at death. In: Forensic science international. Volume 29, Numbers 3-4, 1985, pp. 259-267, PMID 4076954 .
- ^ S. Ohtani, T. Yamamoto: Strategy for the estimation of chronological age using the aspartic acid racemization method with special reference to coefficient of correlation between D / L ratios and ages. In: Journal of forensic sciences. Volume 50, Number 5, 2005, pp. 1020-1027, PMID 16225206 . (Review).
- ↑ JL Bada, B. Herrmann et al: Amino acid racemization in bone and the boiling of the German Emperor Lothar I. In: Applied Geochemistry. Volume 4, Number 3, 1989, pp. 325-327, doi: 10.1016 / 0883-2927 (89) 90036-X .
- ↑ a b Chris McManus: Right Hand, Left Hand - The Origins of Asymmetry in Brains, Bodies, Atoms and Cultures. Harvard University Press, 2004, ISBN 0-674-01613-0 , p. 130 ( limited preview in Google book search).
- ↑ PM Masters, M. Friedman: Racemization of amino acids in alkali-treated food proteins. In: Journal of agricultural and food chemistry. Volume 27, Number 3, 1979 May-Jun, pp. 507-511, PMID 447924 .
- ↑ JL Bada: Kinetics of racemization of amino acids as a function of pH. In: Journal of the American Chemical Society. Volume 94, Number 4, February 1972, pp. 1371-1373, PMID 5060280 .
- ^ H. Frank, W. Woiwode et al .: Determination of the rate of acidic catalyzed racemization of protein amino acids. In: Liebigs Ann Chem. Number 3, 1981, pp. 354-365. doi: 10.1002 / jlac.198119810303 .
- ↑ T. Geiger, S. Clarke: Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. In: The Journal of biological chemistry. Volume 262, Number 2, January 1987, pp. 785-794, PMID 3805008 .
- ↑ a b c d e f g Thorsten Erbe: The quantification of amino acid isomers in foods by means of chiral gas chromatography-mass spectrometry with regard to the relevance and the mechanisms of formation of D-amino acids. Dissertation, Justus Liebig University Giessen, 1999.
- ^ A. Paquet, M. Ching-Yung: Racemization assessment in alkali treated dietary proteins using high-performance liquid chromatography. In: Nutrition Research. Volume 9, Number 9, 1989, pp. 1053-1065. doi: 10.1016 / S0271-5317 (89) 80066-1 .
- ↑ a b c M. Friedman: Chemistry, nutrition, and microbiology of D-amino acids. In: Journal of agricultural and food chemistry. Volume 47, Number 9, September 1999, pp. 3457-3479, PMID 10552672 . (Review).
- ^ JP Richard, TL Amyes: Proton transfer at carbon. In: Current opinion in chemical biology. Volume 5, Number 6, December 2001, pp. 626-633, PMID 11738171 . (Review).
- ↑ JP Richard, TL Amyes: On the importance of being zwitterionic: enzymatic catalysis of decarboxylation and deprotonation of cationic carbon. In: Bioorganic Chemistry. Volume 32, Number 5, October 2004, pp. 354-366, doi: 10.1016 / j.bioorg.2004.05.002 . PMID 15381401 . (Review).
- ↑ a b c d Daniel Björn Stein: Substrate specificity and functionality of epimerization domains in nonribosomal peptide synthesis. Dissertation, Philipps University Marburg, 2006, p. 29.
- ↑ S. Glavas, ME Tanner: Active site residues of glutamate racemase. In: Biochemistry. Volume 40, Number 21, May 2001, pp. 6199-6204, PMID 11371180 .
- ↑ LM Fisher, JG Belasco et al. a .: Energetics of proline racemase: transition-state fractionation factors for the two protons involved in the catalytic steps. In: Biochemistry. Volume 25, Number 9, May 1986, pp. 2543-2551, PMID 3521738 .
- ↑ Geoffrey Zubay: Origins of Life: On Earth and in the Cosmos. Academic Press, 2000, ISBN 0-12-781910-X , p. 296 ( limited preview in Google book search).
- ↑ a b c J. J. Corrigan: D-amino acids in animals. In: Science. Volume 164, Number 3876, April 1969, pp. 142-149, PMID 5774186 .
- ↑ E. Abderhalden : Ferment research. Volume 16-17, S. Hirzel, 1942, p. 301.
- ↑ HA cancer: studies on the metabolism of amino acids in the animal body. In: Hoppe-Seyler's journal for physiological chemistry. Volume 217, 1933, p. 191.
- ↑ a b H. A. Krebs: Metabolism of amino acids: Deamination of amino acids. In: The Biochemical journal. Volume 29, Number 7, July 1935, pp. 1620-1644, PMID 16745832 . PMC 1266672 (free full text).
- ↑ a b H. Blaschko, J. Hawkins: D-Amino acid oxidase in the molluscan liver. In: The Biochemical journal. Volume 52, Number 2, October 1952, pp. 306-310, PMID 13018226 . PMC 1197987 (free full text).
- ↑ F. Ehrlich : About the asymmetrical and symmetrical action of yeast on racemic compounds of naturally occurring amino acids. In: Biochem Z. 63, 1914, pp. 379-401.
- ↑ EO von Lippmann : About the occurrence of leucine and tyrosine in beet molasses. In: Ber Dtsch Chem Ges. Volume 17, 1994, pp. 2835-2840. doi: 10.1002 / cber.188401702243 .
- ↑ S. Fraenkel, H. Gallia, A. Liebster, S. Rosen: About the products of prolonged tryptic digestion of casein. In: Biochem Z. Volume 145, 1924, pp. 225-241.
- ↑ E. Winterstein, C. Reuter and R. Korolew: About the chemical composition of some fungi and about the products that occur during autolysis. In: Landw Versuchsstat. LXXIX - LXX, 1913, pp. 541-562.
- ↑ JH Birkinshaw, H. Raistrick, G. Smith: Studies in the biochemistry of micro-organisms: Fumaryl-dl-alanine (fumaromono-dl-alanide), a metabolic product of Penicillium resticulosum sp.nov. In: The Biochemical journal. Volume 36, Numbers 10-12, December 1942, pp. 829-835, PMID 16747516 . PMC 1266878 (free full text).
- ↑ T. Robinson: D-amino acids in higher plants. In: Life sciences. Volume 19, Number 8, October 1976, pp. 1097-1102, PMID 792607 . (Review).
- ↑ JL Frahn, RJ Illman: The occurrence of D-alanine and D-alanyl-D-alanine in Phalaris tuberosa. In: Phytochem Volume 14, 1975, pp. 1464-1465. doi: 10.1016 / S0031-9422 (00) 98674-6 .
- ↑ Y. Gogami, K. Ito et al. a .: Occurrence of D-serine in rice and characterization of rice serine racemase. In: Phytochemistry. Volume 70, Number 3, February 2009, pp. 380-387, doi: 10.1016 / j.phytochem.2009.01.003 . PMID 19249065 .
- ↑ T. Ogawa, M. Fukuda, K. Sasaoka: Occurrence of N-malonyl-D-alanine in pea seedlings. In: Biochimica et Biophysica Acta . Volume 297, Number 1, January 1973, pp. 60-69, PMID 4144329 .
- ↑ H. Brückner, S. Haasmann, A. Friedrich: Quantification of D-amino acids in human urine using GC-MS and HPLC. In: Amino Acids. Volume 6, 1994, pp. 205-211. doi: 10.1007 / BF00805848 .
- ↑ EE Snell, BM Guirard: Some Interrelationships of Pyridoxine, Alanine and Glycine in Their Effect on Certain Lactic Acid Bacteria. In: PNAS. Volume 29, Number 2, 1943, pp. 66-73, PMID 16588604 . PMC 1078561 (free full text).
- ↑ J. Olivard, EE Snell: Growth and enzymatic activities of vitamin B6 analogues. I. D-Alanine synthesis. In: The Journal of biological chemistry. Volume 213, Number 1, March 1955, pp. 203-214, PMID 14353919 . PMC 1078561 (free full text).
- ↑ EE Snell: The vitamin B6 group: VII. Replacement of vitamin B6 for some microorganisms by d (-) - alanine and an unidentified factor from casein. In: J Biol Chem. Volume 158, 1945, pp. 497-503.
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. John Wiley & Sons, 2003, ISBN 3-906390-29-2 , p. 347 ( limited preview in Google book search).
- ^ WA Wood, IC Gunsalus: D-Alanine formation; a racemase in Streptococcus faecalis. In: The Journal of biological chemistry. Volume 190, Number 1, May 1951, pp. 403-416, PMID 14841188 .
- ↑ J. Ju, H. Misono, K. Ohnishi: Directed evolution of bacterial alanine racemases with higher expression level. In: Journal of bioscience and bioengineering. Volume 100, Number 3, September 2005, pp. 246-254, doi: 10.1263 / jbb.100.246 . PMID 16243272 .
- ^ RJ Thompson, HG Bouwer et al. a .: Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth. In: Infection and Immunity. Volume 66, Number 8, August 1998, pp. 3552-3561, PMID 9673233 . PMC 108386 (free full text).
- ↑ D. Billot-Klein, L. Gutmann et al. a .: Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. In: Journal of bacteriology. Volume 176, Number 8, April 1994, pp. 2398-2405, PMID 8157610 . PMC 205365 (free full text).
- ↑ PE Reynolds, HA Snaith et al. a .: Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. In: The Biochemical journal. Volume 301, July 1994, pp. 5-8, PMID 8037690 . PMC 1137133 (free full text).
- ^ CA Arias, M. Martín-Martinez u. a .: Characterization and modeling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. In: Molecular microbiology. Volume 31, Number 6, March 1999, pp. 1653-1664, PMID 10209740 .
- ^ A b Norma Christine Stäbler: Investigations on the formation of D-amino acids with Corynebacterium glutamicum. Dissertation, Heinrich Heine University Düsseldorf, 2010, p. 7.
- ↑ MF Freeman, C. Gurgui u. a .: Metagenome Mining Reveals Polytheonamides as Posttranslationally Modified Ribosomal Peptides. In: Science. [electronic publication before printing] September 2012, doi: 10.1126 / science.1226121 . PMID 22983711 .
- ↑ T. Hamada, S. Matsunaga et al. a .: Solution structure of polytheonamide B, a highly cytotoxic nonribosomal polypeptide from marine sponge. In: Journal of the American Chemical Society. Volume 132, number 37, September 2010, pp. 12941-12945, doi: 10.1021 / ja104616z . PMID 20795624 .
- ↑ D. Ackermann , M. Mohr: About nitrogenous components of the liver of the shark (Acanthias vulgaris). In: Z Biol. Volume 98, number 37, 1937, p. 26.
- ^ LR Lyle, JW Jutila: D-amino acid oxidase induction in the kidneys of germ-free mice. In: Journal of bacteriology. Volume 96, Number 3, September 1968, pp. 606-608, PMID 4389707 . PMC 252348 (free full text).
- ↑ JL Auclair, RL Patton: On the occurrence of D-Alanine in the haemolymph of the milkweed bug, Oncopeltus fasciatus. In: Revue canadienne de biologie. Volume 9, Number 1, April 1950, pp. 3-8, PMID 15417891 .
- ↑ a b Gianluca Molla, Luciano Piubelli and others: Enzymatic Detection of D-Amino Acids. In: Loredano Pollegioni, Stefano Servi (Ed.): Unnatural Amino Acids. Volume 794, 2012, ISBN 978-1-61779-330-1 , pp. 273-289. doi : 10.1007 / 978-1-61779-331-8_18 .
- ↑ NG Srinivasan, JJ Corrigan, A. Meister: Biosynthesis of D-Serine in the silkworm, Bombyx mori. (PDF; 665 kB) In: The Journal of biological chemistry. Volume 240, February 1965, pp. 796-800, PMID 14275137 .
- ↑ JJ Corrigan, NG Srinivasan: The occurrence of certain D-amino acids in insects. In: Biochemistry. Volume 5, Number 4, April 1966, pp. 1185-1190, PMID 5958195 .
- ↑ Y. Nagata, K. Yamamoto et al. a .: The presence of free D-alanine, D-proline and D-serine in mice. In: Biochimica et Biophysica Acta . Volume 1115, Number 3, January 1992, pp. 208-211, PMID 1346751 .
- ↑ P. Melchiorri, L. Negri: The dermorphin peptide family. In: General pharmacology. Volume 27, Number 7, October 1996, pp. 1099-1107, PMID 8981054 . (Review).
- ↑ A. Anastasi, V. Erspamer, JM Cei: Isolation and amino acid sequence of physalaemin, the main active polypeptide of the skin of Physalaemus fuscumaculatus. In: Archives of biochemistry and biophysics. Volume 108, November 1964, pp. 341-348, PMID 14240587 .
- ↑ Rebecca Jo Jackway: Biologically Active Peptides from Australian Amphibians. PhD Thesis, University of Adelaide, 2008, p. 165.
- ↑ literally: At this time there is no conclusive evidence for the occurrence of D-amino acids in the proteins of plants and animals. Alton Meister: Biochemistry of the amino acids Academic Press, 1965.
- ↑ M. Broccardo, V. Erspamer a. a .: Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin. In: British journal of pharmacology. Volume 73, Number 3, July 1981, pp. 625-631, PMID 7195758 . PMC 2071698 (free full text).
- ^ V. Erspamer, P. Melchiorri u. a .: Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. In: PNAS. Volume 86, Number 13, July 1989, pp. 5188-5192, PMID 2544892 . PMC 297583 (free full text).
- ↑ M. Amiche, A. Delfour, P. Nicolas: Opiod peptides from frog skin. In: Pierre Jollès (Ed.): D-Amino Acids in Sequences of Secreted Peptides of Multicellular Organisms. Springer, 1998, ISBN 3-7643-5814-9 , pp. 57–72 ( limited preview in the Google book search).
- ↑ LH Lazarus, M. Attila: The toad, ugly and venomous, wears yet a precious jewel in his skin. In: Progress in neurobiology. Volume 41, Number 4, October 1993, pp. 473-507, PMID 8210414 . (Review).
- ^ G. Kreil: Peptides containing a D-amino acid from frogs and molluscs. In: The Journal of biological chemistry. Volume 269, Number 15, April 1994, pp. 10967-10970, PMID 8157620 . (Review).
- ↑ SD Heck, WS Faraci et al. a .: Posttranslational amino acid epimerization: enzyme-catalyzed isomerization of amino acid residues in peptide chains. In: PNAS. Volume 93, Number 9, April 1996, pp. 4036-4039, PMID 8633012 . PMC 39482 (free full text).
- ↑ R. Liardon, R. Jost: racemization of free and protein-bound amino acids in strong mineral acid. In: International journal of peptide and protein research. Volume 18, Number 5, November 1981, pp. 500-505, PMID 7341532 .
- ↑ H. Brückner, T. Westhauser, H. Godel: Liquid chromatographic determination of D- and L-amino acids by derivatization with o-phthaldialdehyde and N-isobutyryl-L-cysteine. Applications with reference to the analysis of peptidic antibiotics, toxins, drugs and pharmaceutically used amino acids. In: Journal of chromatography. A. Volume 711, Number 1, September 1995, pp. 201-215, PMID 7496491 .
- ↑ RH Buck, K. Krummen: High-performance liquid chromatographic determination of enantiomeric amino acids and amino alcohols after derivatization with o-phthaldialdehyde and various chiral mercaptans. Application to peptide hydrolysates. In: Journal of chromatography. Volume 387, January 1987, pp. 255-265, PMID 3558624 .
- ↑ A. Hashimoto, T. Nishikawa et al. a .: The presence of free D-serine in rat brain. In: FEBS letters. Volume 296, Number 1, January 1992, pp. 33-36, PMID 1730289 .
- ^ A b N. W. Kleckner, R. Dingledine: Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. In: Science. Volume 241, Number 4867, August 1988, pp. 835-837, PMID 2841759 .
- ↑ a b H. Wolosker, S. Blackshaw, SH Snyder: Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. In: PNAS. Volume 96, Number 23, November 1999, pp. 13409-13414, PMID 10557334 . PMC 23961 (free full text).
- ↑ H. Wolosker, E. Dumin et al. a .: D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. In: The FEBS journal. Volume 275, Number 14, July 2008, pp. 3514-3526, doi: 10.1111 / j.1742-4658.2008.06515.x . PMID 18564180 . (Review).
- ↑ S. Sacchi, M. Bernasconi and a .: pLG72 modulates intracellular D-serine levels through its interaction with D-amino acid oxidase: effect on schizophrenia susceptibility. In: The Journal of biological chemistry. Volume 283, Number 32, 2008, pp. 22244-22256, doi: 10.1074 / jbc.M709153200 . PMID 18544534 .
- ↑ HJ Ryu, JE Kim et al. a .: Potential roles of D-serine and serine racemase in experimental temporal lobe epilepsy. In: Journal of neuroscience research. Volume 88, number 11, 2010, pp. 2469-2482, doi: 10.1002 / jnr . 22415 . PMID 20623543 .
- ^ SA Fuchs, R. Berger, TJ de Koning: D-serine: the right or wrong isoform? In: Brain research. Volume 1401, July 2011, pp. 104-117, doi: 10.1016 / j.brainres.2011.05.039 . PMID 21676380 . (Review).
- ↑ Julia Scharlau: Studies on the effects of adolescent chronic cannabinoid treatment on a mouse model of schizophrenia. (PDF; 827 kB) Dissertation, Friedrich-Wilhelms-Universität Bonn, 2012, p. 16.
- ↑ E. Kartvelishvily, M. Shleper et al. a .: Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. In: The Journal of biological chemistry. Volume 281, Number 20, May 2006, pp. 14151-14162, doi: 10.1074 / jbc.M512927200 . PMID 16551623 .
- ↑ K. Miya, R. Inoue et al. a .: Serine racemase is predominantly localized in neurons in mouse brain. In: The Journal of comparative neurology. Volume 510, Number 6, October 2008, pp. 641-654, doi: 10.1002 / cne.21822 . PMID 18698599 .
- ↑ L. Pollegioni, S. Sacchi: Metabolism of the neuromodulator D-serine. In: Cellular and molecular life sciences. Volume 67, Number 14, July 2010, pp. 2387-2404, doi: 10.1007 / s00018-010-0307-9 . PMID 20195697 . (Review).
- ↑ JT Kantrowitz, DC Javitt: N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? In: Brain research bulletin. Volume 83, number 3–4, September 2010, pp. 108–121, doi: 10.1016 / j.brainresbull.2010.04.006 . PMID 20417696 . PMC 2941541 (free full text). (Review).
- ^ JT Coyle: Glutamate and schizophrenia: beyond the dopamine hypothesis. In: Cellular and molecular neurobiology. Volume 26, Numbers 4-6, 2006 Jul-Aug, pp. 365-384, doi: 10.1007 / s10571-006-9062-8 . PMID 16773445 . (Review).
- ↑ I. Chumakov, M. Blumenfeld a. a .: Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. In: PNAS. Volume 99, Number 21, October 2002, pp. 13675-13680, doi: 10.1073 / pnas.182412499 . PMID 12364586 . PMC 129739 (free full text).
- ^ A. Corvin, KA McGhee et al. a .: Evidence for association and epistasis at the DAOA / G30 and D-amino acid oxidase loci in an Irish schizophrenia sample. In: American journal of medical genetics. Volume 144B, Number 7, October 2007, pp. 949-953, doi: 10.1002 / ajmg.b.30452 . PMID 17492767 .
- ↑ T. Ohnuma, N. Shibata et al. a .: Association analysis of glycine- and serine-related genes in a Japanese population of patients with schizophrenia. In: Progress in neuro-psychopharmacology & biological psychiatry. Volume 33, Number 3, April 2009, pp. 511-518, doi: 10.1016 / j.pnpbp.2009.02.004 . PMID 19223009 .
- ↑ K. Hashimoto, T. Fukushima et al. a .: Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. In: Archives of general psychiatry. Volume 60, Number 6, June 2003, pp. 572-576, doi: 10.1001 / archpsyc.60.6.572 . PMID 12796220 .
- ↑ I. Bendikov, C. Nadri et al. a .: A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. In: Schizophrenia research. Volume 90, number 1–3, February 2007, pp. 41–51, doi: 10.1016 / j.schres.2006.10.010 . PMID 17156977 .
- ↑ K. Hashimoto, G. Engberg et al. a .: Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. In: Progress in neuro-psychopharmacology & biological psychiatry. Volume 29, Number 5, June 2005, pp. 767-769, doi: 10.1016 / j.pnpbp.2005.04.023 . PMID 15939521 .
- ↑ L. Verrall, M. Walker et al. a .: d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. In: The European journal of neuroscience. Volume 26, Number 6, September 2007, pp. 1657-1669, doi: 10.1111 / j.1460-9568.2007.05769.x . PMID 17880399 . PMC 2121142 (free full text).
- ↑ L. Verrall, PW Burnet et al. a .: The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. In: Molecular psychiatry. Volume 15, Number 2, February 2010, pp. 122-137, doi: 10.1038 / mp.2009.99 . PMID 19786963 . PMC 2811712 (free full text). (Review).
- ↑ R. Kapoor, KS Lim et al. a .: Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1). In: Brain research. Volume 1106, number 1, August 2006, pp. 205-210, doi: 10.1016 / j.brainres.2006.05.082 . PMID 16828464 .
- ↑ C. Madeira, ME Freitas et al. a .: Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. In: Schizophrenia research. Volume 101, number 1-3, April 2008, pp. 76-83, doi: 10.1016 / j.schres.2008.02.002 . PMID 18378121 .
- ^ JT Coyle, G. Tsai, DC Goff: Ionotropic glutamate receptors as therapeutic targets in schizophrenia. In: Current drug targets. Volume 1, Number 2, April 2002, pp. 183-189, PMID 12769626 . (Review).
- ↑ G. Tsai, P. Yang et al. a .: D-serine added to antipsychotics for the treatment of schizophrenia. In: Biological psychiatry. Volume 44, Number 11, December 1998, pp. 1081-1089, PMID 9836012 .
- ↑ HJ Tuominen, J. Tiihonen, K. Wahlbeck: Glutamatergic drugs for schizophrenia. In: Cochrane database of systematic reviews (online). Number 2, 2006, S. CD003730, doi: 10.1002 / 14651858.CD003730.pub2 . PMID 16625590 . (Review).
- ↑ DV Ferraris, T. Tsukamoto: Recent advances in the discovery of D-amino acid oxidase inhibitors and their therapeutic utility in schizophrenia. In: Current pharmaceutical design. Volume 17, Number 2, 2011, pp. 103-111, PMID 21361869 . (Review).
- ↑ CA Strick, C. Li et al. a .: Modulation of NMDA receptor function by inhibition of D-amino acid oxidase in rodent brain. In: Neuropharmacology. Volume 61, number 5-6, 2011 Oct-Nov, pp. 1001-1015, doi: 10.1016 / j.neuropharm.2011.06.029 . PMID 21763704 .
- ↑ T. Sparey, P. Abeywickrema et al. a .: The discovery of fused pyrrole carboxylic acids as novel, potent D-amino acid oxidase (DAO) inhibitors. In: Bioorganic & medicinal chemistry letters. Volume 18, Number 11, June 2008, pp. 3386-3391, doi: 10.1016 / j.bmcl.2008.04.020 . PMID 18455394 .
- ↑ Berry JF, Ferraris DV, Duvall B, et al. : Synthesis and SAR of 1-hydroxy-1H-benzo [d] imidazol-2 (3H) -ones as Inhibitors of D-Amino Acid Oxidase . In: ACS Med Chem Lett . 3, No. 10, 2012, pp. 839-843. doi : 10.1021 / ml300212a . PMID 23243487 .
- ↑ Sacchi S, Rosini E, Pollegioni L, Molla G: D-Amino Acid Oxidase Inhibitors as a Novel Class of Drugs for Schizophrenia Therapy . In: Curr. Pharm. Des. . 2012. PMID 23116391 .
- ↑ Paul P, de Belleroche J: The role of D-amino acids in amyotrophic lateral sclerosis pathogenesis: a review . In: Amino Acids . 43, No. 5, 2012, pp. 1823-1831. doi : 10.1007 / s00726-012-1385-9 . PMID 22890612 .
- ↑ Sasabe J, Chiba T, Yamada M, et al. : D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis . In: EMBO J. . 26, No. 18, 2007, pp. 4149-4159. doi : 10.1038 / sj.emboj.7601840 . PMID 17762863 . PMC 2230675 (free full text).
- ↑ DS Dunlop, A. Neidle et al. a .: The presence of free D-aspartic acid in rodents and man. In: Biochemical and biophysical research communications. Volume 141, Number 1, November 1986, pp. 27-32, PMID 3801000 .
- ↑ H. Wolosker, A. D'Aniello, SH Snyder: D-aspartate disposition in neuronal and endocrine tissues: ontogeny, biosynthesis and release. In: Neuroscience. Volume 100, Number 1, 2000, pp. 183-189, PMID 10996468 .
- ↑ M. Katane, H. Homma: D-aspartate oxidase: the sole catabolic enzyme acting on free D-aspartate in mammals. In: Chemistry & biodiversity. Volume 7, number 6, June 2010, pp. 1435–1449, doi: 10.1002 / cbdv.200900250 . PMID 20564562 . (Review).
- ↑ a b H. Ohide, Y. Miyoshi et al. a .: D-Amino acid metabolism in mammals: biosynthesis, degradation and analytical aspects of the metabolic study. In: Journal of chromatography. B Volume 879, number 29, November 2011, pp. 3162-3168, doi: 10.1016 / j.jchromb.2011.06.028 . PMID 21757409 . (Review).
- ↑ a b P. M. Kim, X. Duan et al. a .: Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. In: PNAS. Volume 107, Number 7, February 2010, pp. 3175-3179, doi: 10.1073 / pnas.0914706107 . PMID 20133766 . PMC 2840285 (free full text).
- ↑ Y. Mori, K. Aki et al. a .: UV B-irradiation enhances the racemization and isomerization of aspartyl residues and production of N? -carboxymethyl lysine (CML) in keratin of skin. In: Journal of chromatography B. Volume 879, number 29, November 2011, pp. 3303-3309, doi: 10.1016 / j.jchromb.2011.05.010 . PMID 21636332 .
- ↑ N. Fujii, Y. Kaji et al. a .: Collapse of homochirality of amino acids in proteins from various tissues during aging. In: Chemistry & biodiversity. Volume 7, number 6, June 2010, pp. 1389-1397, doi: 10.1002 / cbdv.200900337 . PMID 20564552 . (Review).
- ↑ N. Fujii: D-amino acid in elderly tissues. In: Biological & pharmaceutical bulletin. Volume 28, Number 9, September 2005, pp. 1585-1589, PMID 16141520 . (Review).
- ↑ Graham C. Barrett, Donald Trevor Elmore: Amino Acids and Peptides. Cambridge University Press, 1998, ISBN 0-521-46292-4 , pp. 15-16 ( limited preview in Google Book Search).
- ↑ S. Ritz-Timme , MJ Collins: Racemization of aspartic acid in human proteins. In: Aging research reviews. Volume 1, Number 1, February 2002, pp. 43-59, PMID 12039448 . (Review).
- ↑ H. Mori, K. Ishii et al. a .: Racemization: its biological significance on neuropathogenesis of Alzheimer's disease. In: The Tohoku journal of experimental medicine. Volume 174, Number 3, November 1994, pp. 251-262, PMID 7761990 .
- ^ R. Shapira, GE Austin, SS Mirra: Neuritic plaque amyloid in Alzheimer's disease is highly racemized. In: Journal of neurochemistry. Volume 50, Number 1, January 1988, pp. 69-74, PMID 3121789 .
- ↑ AE Roher, JD Lowenson et al. a .: Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. In: The Journal of biological chemistry. Volume 268, Number 5, February 1993, pp. 3072-3083, PMID 8428986 .
- ↑ a b T. Tomiyama, S. Asano et al. a .: Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. In: The Journal of biological chemistry. Volume 269, Number 14, April 1994, pp. 10205-10208, PMID 8144598 .
- ^ Patrick R. Hof, Charles V. Mobbs: Handbook of the Neuroscience of Aging. Academic Press, 2009, ISBN 0-12-374898-4 , p. 41 ( limited preview in Google Book Search).
- ↑ ML Moro, MJ Collins, E. Cappellini: Alzheimer's disease and amyloid beta-peptide deposition in the brain: a matter of 'aging'? In: Biochemical Society transactions. Volume 38, Number 2, April 2010, pp. 539-544, doi: 10.1042 / BST0380539 . PMID 20298218 . (Review).
- ↑ Günter Westphal, Gerhard Gerber, Bodo Lipke: Proteins - Nutritive and functional properties. Springer, 2003 ISBN 3-540-00232-4 , p. 125 ( limited preview in the Google book search).
- ^ Gerhard G. Habermehl , Peter E. Hammann and others: Natural substance chemistry. 3rd edition, Springer, 2008, ISBN 3-540-73732-4 , p. 252 ( limited preview in the Google book search).
- ↑ NJ Benevenga, RD Steele: Adverse effects of excessive consumption of amino acids. In: Annual review of nutrition. Volume 4, 1984, pp. 157-181, doi: 10.1146 / annurev.nu.04.070184.001105 . PMID 6235826 . (Review).
- ↑ J. Zagon, L.-I. Dehne, K.-W. Bögl: D-Amino acids in organisms and food. In: Nutr Res. Vol. 14, 1994, pp. 445-463.
- ↑ Werner Baltes , Reinhard Matissek : Lebensmittelchemie 7th edition, Springer, 2011, ISBN 3-642-16538-9 , p. 164 ( limited preview in the Google book search).
- ↑ W. Leuchtenberger, K. Huthmacher, K. Drauz: Biotechnological production of amino acids and derivatives: current status and prospects. In: Applied Microbiology and Biotechnology . Volume 69, Number 1, November 2005, pp. 1-8, doi: 10.1007 / s00253-005-0155-y . PMID 16195792 . (Review).
- ^ FH Leibach, V. Ganapathy: Peptide transporters in the intestine and the kidney. In: Annual review of nutrition. Volume 16, 1996, pp. 99-119, doi: 10.1146 / annurev.nu.16.070196.000531 . PMID 8839921 . (Review).
- ↑ a b c M. Friedman: Origin, microbiology, nutrition, and pharmacology of D-amino acids. In: Chemistry & biodiversity. Volume 7, number 6, 2010, pp. 1491-1530, doi: 10.1002 / cbdv.200900225 . PMID 20564567 . (Review).
- ^ W. Heine, K. Wutzke, U. Drescher: D-amino acid utilization in infants measured with the 15N-tracer technique. In: Clinical nutrition. Volume 2, Number 1, April 1983, pp. 31-35, PMID 16829405 .
- ↑ M. Friedman R. Liardon: racemization kinetics of amino acid residues in alkali-treated soybean protein. In: J Agric Food Chem. Volume 33, 1985, pp. 666-672.
- ^ JC Crawhall, DF Elliott: A note on the racemization of serine. In: The Biochemical journal. Volume 48, Number 2, February 1951, pp. 237-238, PMID 14820833 . PMC 1275510 (free full text).
- ^ M. Lüpke, H. Brückner: Gas chromatographic evaluation of amino acid epimerization in the course of gelatin manufacturing and processing. In: Journal for Food Investigation and Research A. Volume 206, Number 5, 1998, pp. 323–328. doi: 10.1007 / s002170050266 .
- ↑ Nutrition Report 1996. German Society for Nutrition , 1996, ISBN 3-921606-33-0 , S. 149th
- ^ A b c Johannes Friedrich Diehl : Chemistry in food. John Wiley & Sons, 2012, ISBN 3-527-66084-4 , p. 95 ( limited preview in Google book search).
- ↑ H. Lapierre, G. Holtrop and a .: Is D-methionine bioavailable to the dairy cow? In: Journal of dairy science. Volume 95, number 1, January 2012, pp. 353-362, doi: 10.3168 / jds.2011-4553 . PMID 22192214 .
- ^ WH Fishman, C. Artom: Serine injury. In: J Biol Chem. Volume 145, 1942, pp. 345-346.
- ^ RP Morehead, C. Artom, WH Fishman: The nephrotoxic action of serine. In: Proceedings. American Federation for Clinical Research. Volume 2, 1945, p. 81, PMID 20275626 .
- ↑ FA Carone, CE Ganote: D-serine nephrotoxicity. The nature of proteinuria, glucosuria, and aminoaciduria in acute tubular necrosis. In: Archives of pathology. Volume 99, Number 12, December 1975, pp. 658-662, PMID 1203037 .
- ↑ RE Williams, EA Lock: D-serine-induced nephrotoxicity: possible interaction with tyrosine metabolism. In: Toxicology. Volume 201, number 1-3, September 2004, pp. 231-238, doi: 10.1016 / j.tox.2004.05.001 . PMID 15297036 .
- ^ DR Peterson, FA Carone: Renal regeneration following d-serine induced acute tubular necrosis. In: The Anatomical record. Volume 193, Number 3, March 1979, pp. 383-388, doi: 10.1002 / ar.1091930305 . PMID 426302 .
- ↑ CE Ganote, DR Peterson, FA Carone: The nature of D-serine-induced nephrotoxicity. In: The American journal of pathology. Volume 77, Number 2, November 1974, pp. 269-282, PMID 4447130 . PMC 1910915 (free full text).
- ↑ MS Pilone, L. Pollegioni: D-amino acid oxidase as an industrial biocatalyst. In: Biocatalysis and Biotransformation. Volume 20, Number 3, 2002, pp. 145-159. doi: 10.1080 / 10242420290020679 .
- ↑ AW Krug, K. Völker u. a .: Why is D-serine nephrotoxic and alpha-aminoisobutyric acid protective? ( Memento of the original from January 16, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: American Journal of Physiology - Renal physiology. Volume 293, number 1, 2007, pp. F382-F390, doi: 10.1152 / ajprenal.00441.2006 . PMID 17429029 .
- ↑ G. Lubec, C. Wolf, B. Bartosch: Aminoacid isomerization and microwave exposure. In: Lancet. Volume 334, Number 8676, December 1989, pp. 1392-1393, PMID 2574327 .
- ^ Karl S. Kruszelnicki: Microwaves damage food. In: ABC abcscience. 23 March 2006
- ^ W. Segal: Microwave heating of milk. In: Lancet. Volume 335, Number 8687, February 1990, p. 470, PMID 1968186 .
- ↑ The effect of water irradiated with microwaves on plants. Retrieved August 24, 2012.
- ↑ Dangerous microwaves. October 24, 2008, accessed September 28, 2012.
- ↑ P. Fritz, LI Dehne, J. Zagon, KW Bögl: On the question of amino acid isomerization in the microwave field: Results of a model experiment with standard solutions. In: Journal of Nutritional Science. Volume 31, Number 3, 1992, pp. 219-224.
- ↑ LI Dehne, P. Fritz: Isomerization of Amino Acids by Microwave Heating? In: Federal Health Gazette. Volume 35, 1992, pp. 463-464.
- ↑ P. Sieber, P. Eberhardt, PU Gallmann: Heat treatment of milk in domestic microwave ovens. In: Int Dairy Journal. Volume 6, 1996, pp. 231-246, doi: 10.1016 / 0958-6946 (95) 00009-7 .
- ↑ J. Zagon, L. Dehne, K.-W. Bögl: Isomerization of amino acids in food. Part I: Mechanisms and distribution of amino acid isomerization in organisms and foods. In: Nutrition review. Volume 38, 1991, pp. 275-278 and 324-328.
- ↑ A. Cherkin, JL Davis, MW Garman: D-Proline: stereospecificity and sodium chloride dependence of lethal convulsant activity in the chick. In: Pharmacology, biochemistry, and behavior. Volume 8, Number 5, May 1978, pp. 623-625, PMID 674269 .
- ↑ A. Schieber, H. Brückner u. a .: Evaluation of D-amino acid levels in rat by gas chromatography-selected ion monitoring mass spectrometry: no evidence for subacute toxicity of orally fed D-proline and D-aspartic acid. In: Journal of chromatography. Volume 691, Number 1, March 1997, pp. 1-12, PMID 9140753 .
- ^ SN Ali, G. O'Toole, M. Tyler: Milk bottle burns. In: The Journal of burn care & rehabilitation. Volume 25, Number 5, 2004, pp. 461-462, PMID 15353942 .
- ^ IA Jaffe, K. Altman, P. Merryman: The antipyridoxine effect of penicillamine in man. In: The Journal of clinical investigation. Volume 43, October 1964, pp. 1869-1873, doi: 10.1172 / JCI105060 . PMID 14236210 . PMC 289631 (free full text).
- ↑ TN Schumacher, LM Mayr u. a .: Identification of D-peptide ligands through mirror-image phage display. In: Science. Volume 271, Number 5257, March 1996, pp. 1854-1857, PMID 8596952 .
- ↑ Katja Wiesehan: Identification and characterization of a specific ligand for the Alzheimer's amyloid-β-peptide (Aβ). (PDF; 11.1 MB) Dissertation, University of Bayreuth, 2003, p. 21.
- ↑ JM Manning, S. Moore: Determination of D- and L-amino acids by ion exchange chromatography as LD and LL dipeptides. In: The Journal of biological chemistry. Volume 243, Number 21, November 1968, pp. 5591-5597, PMID 5699053 .
- ↑ K. Soda: Microdetermination of D-amino acids and D-amino acid oxidase activity with 3, methyl-2-benzothiazolone hydrazone hydrochloride. In: Analytical biochemistry. Volume 25, Number 1, October 1968, pp. 228-235, PMID 4387536 .
- ↑ doi: 10.1002 / ardp.19863190515 .
- ↑ DL Kirschner, TK Green: Separation and sensitive detection of D-amino acids in biological matrices. In: Journal of separation science. Volume 32, Number 13, July 2009, pp. 2305-2318, doi: 10.1002 / jssc.200900101 . PMID 19569111 . (Review).
- ↑ K. Hamase, A. Morikawa et al. a .: Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. In: Analytical sciences. Volume 25, Number 8, August 2009, pp. 961-968, PMID 19667471 . (Review).
- ↑ Clarissa Dorothee Marzini: Limits of the quantitative enantiomer analysis of alpha-amino acids. (PDF; 1.7 MB) Dissertation, Eberhard-Karls-Universität Tübingen, 2007, pp. 20–21.
- ↑ N. Nimura, T. Kinoshita: O-Phthaldialdehyde N-acetyl-L-cysteine as a chiral derivatization reagent for liquid chromatographic optical resolution of amino acid enantiomers and is application to conventional amino acid analysis. In: Journal of Chromatography A. Volume 352, 1986, pp. 169-177. doi: 10.1016 / S0021-9673 (01) 83377-X .
- ↑ Birgit Geueke: New amino acid oxidases from Rhodococcus opacus and Arthrobacter protophormiae: Investigations on biochemical characterization, cloning and expression. (PDF; 9.6 MB) Dissertation, Heinrich Heine University Düsseldorf, 2002, p. 1.
- ↑ E. Takahashi, M. Furui et al. a .: D-lysine production from L-lysine by successive chemical racemization and microbial asymmetric degradation. In: Applied Microbiology and Biotechnology . Volume 47, Number 4, April 1997, pp. 347-351, PMID 9163947 .
- ↑ K. Isobe, H. Tamauchi et al. a .: A Simple Enzymatic Method for Production of a Wide Variety of D-Amino Acids Using L-Amino Acid Oxidase from Rhodococcus sp. AIU Z-35-1. In: Enzyme research. Volume 2010, 2010, p. 567210, doi: 10.4061 / 2010/567210 . PMID 21048866 . PMC 2962901 (free full text).
- ↑ Kerstin Ragnitz: Immobilization and stabilization of hydantoinase and LN-carbamoylase from Arthrobacter aurescens DSM 3747. (PDF; 997 kB) Dissertation, University of Stuttgart, 2000, p. 4.
- ↑ Global D-Amino Acids Market to Reach $ 3.7 Billion by 2017, According to a New Report by Global Industry Analysts, Inc. At: prweb.com, August 25, 2011.
- ↑ S. Martínez-Rodríguez, AI Martínez-Gómez u. a .: Natural occurrence and industrial applications of D-amino acids: an overview. In: Chemistry & biodiversity. Volume 7, number 6, June 2010, pp. 1531–1548, doi: 10.1002 / cbdv.200900245 . PMID 20564568 . (Review).
- ↑ a b Markus Werner: Cloning of D-carbamoylase from Arthrobacter crystallopoietes DSM 20117. Dissertation, University of Stuttgart, 2001.
- ↑ B. Kutscher, M. Bernd: Chemistry and molecular biology in the search for new LHRH antagonists. In: Angew Chem. Volume 109, number 20, 1997, pp. 2240-2254, doi: 10.1002 / ange.19971092005 .
- ↑ T. Beckers, M. Bernd u. a .: Structure-function studies of linear and cyclized peptide antagonists of the GnRH receptor. In: Biochemical and biophysical research communications. Volume 289, Number 3, December 2001, pp. 653-663, doi: 10.1006 / bbrc.2001.5939 . PMID 11726197 .
- ↑ Alaa A.-M. Abdel-Aziz, Yousif A. Asiri and others: Tadalafil. ( Memento of the original from December 24, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 4.4 MB) In: Harry G. Brittain (Ed.): Profiles of Drug Substances, Excipients and Related Methodology. Academic Press, 2011, ISBN 0-12-387702-4 , pp. 287–329 ( limited preview in Google Book Search).
- ↑ E. Fischer, B. Heller and a .: Therapy of depression by phenylalanine. Preliminary note. In: drug research. Volume 25, Number 1, January 1975, p. 132, PMID 1173765 .
- ↑ S. Ehrenpreis: D-phenylalanine and other enkephalinase inhibitors as pharmacological agents: implications for some important therapeutic application. In: Substance and alcohol actions / misuse. Volume 3, Number 4, 1982, pp. 231-239, PMID 6301083 .
- ↑ Mitchell Bebel Stargrove, Jonathan Treasure, Dwight L. McKee: Herb, Nutrient, and Drug Interactions. Elsevier Health Sciences, 2008, ISBN 0-323-02964-7 , p. 682 ( limited preview in Google book search).
- ↑ Phenylalanine. University of Maryland Medical Center, accessed September 21, 2012.
- ↑ DL Williams: A veterinary approach to the European honey bee (Apis mellifera) In: Veterinary journal. Volume 160, number 1, July 2000, pp. 61-73, doi: 10.1053 / tvjl.2000.0474 . PMID 10950136 . (Review).