Isoleucine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
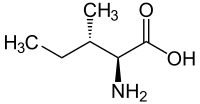
|
||||||||||||||||||||||
|
L -Isoleucine For structures of other isomers see stereoisomerism |
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Isoleucine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 13 NO 2 | |||||||||||||||||||||
| Brief description |
colorless solid with a faint odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 131.18 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
284 ° C (decomposition, L -isoleucine) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
soluble in water (40 g l −1 at 20 ° C, L -isoleucine) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Isoleucine , abbreviated Ile or I , is in its natural L -form an essential proteinogenic α - amino acid .
Since isoleucine can be derived from aspartic acid , it belongs to the aspartate group . It belongs together with its structural isomers leucine , norleucine and tert -leucine for substance group of leucine .
history
In 1901, Emil Fischer , who later won the Nobel Prize , suggested that the leucine fraction he isolated contained, in addition to leucine, an "amino acid of the same composition but with a stronger rotation". In fact, in 1903 the German chemist Felix Ehrlich was able to isolate a compound isomeric to leucine from the molasses of beet sugar , called isoleucine. Felix Ehrlich recognized the constitution in 1907 through further investigation.
Occurrence
Isoleucine is a peptide- bound component of animal and vegetable proteins . The following examples each relate to 100 g of the foodstuff; the percentage of isoleucine in the total protein is also given.
| Food | protein | Isoleucine | proportion of |
|---|---|---|---|
| beef | 21.26 g | 967 mg | 4.5% |
| Chicken breast fillet | 23.09 g | 1219 mg | 5.3% |
| salmon | 20.42 g | 968 mg | 4.7% |
| Chicken egg | 12.58 g | 672 mg | 5.3% |
| Cow's milk, 3.7% fat | 3.28 g | 198 mg | 6.0% |
| Walnuts | 15.23 g | 625 mg | 4.1% |
| Wholemeal wheat flour | 13.70 g | 508 mg | 3.7% |
| Wholemeal corn flour | 6.93 g | 248 mg | 3.6% |
| Rice, unpeeled | 7.94 g | 336 mg | 4.2% |
| Peas, dried | 24.55 g | 1014 mg | 4.1% |
All of these foods contain almost exclusively chemically bound L -isoleucine as a protein component, but no free L -isoleucine in the raw state .
Stereoisomerism
Isoleucine has two stereocenters, so there are four stereoisomers ; In our environment, however, only L -isoleucine plays a role as a proteinogenic amino acid and is physiologically important. When "isoleucine" is spoken of without any additional name ( descriptor ), L- isoleucine is generally meant.
D -isoleucine is the enantiomer of natural L -isoleucine. L - allo -isoleucine and its enantiomer D - allo -isoleucine are diastereomers of L -isoleucine.
| Isomers of isoleucine | ||||
| Surname | L -isoleucine | D -isoleucine | L - allo -isoleucine | D - allo -isoleucine |
| other names | (2 S , 3 S ) -2-amino-3-methylpentanoic acid ( S ) -isoleucine |
(2 R , 3 R ) -2-amino-3-methylpentanoic acid (2 R , 3 R ) -2-amino-3-methylvaleric acid |
(2 S , 3 R ) -2-amino-3-methylpentanoic acid | (2 R , 3 S ) -2-amino-3-methylpentanoic acid |
| Structural formula |

|

|

|
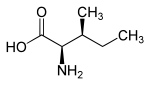
|
| CAS number | 73-32-5 | 319-78-8 | 1509-34-8 | 1509-35-9 |
| 443-79-8 (unspec.) | ||||
| EC number | 200-798-2 | 206-269-2 | 216-142-3 | 216-143-9 |
| 207-139-8 (unspec.) | ||||
| ECHA info card | 100,000,726 | 100.005.701 | 100.014.675 | 100.014.676 |
| 100.006.492 (unspec.) | ||||
| PubChem | 6306 | 76551 | 99288 | 94206 |
| 791 (unspec.) | ||||
| Wikidata | Q484940 | Q27103290 | Q27092902 | Q27109362 |
| Q27117434 (unspec.) | ||||
| Melting point | Decomposition: 284 ° C | |||
properties
Isoleucine is present at the isoelectric point (a certain pH value ) as a zwitterion (inner salt), with the proton of the carboxy group (-COOH) dissociating and the amino group (-NH 2 ) protonating.
- Side chain : lipophilic
- isoelectric point : 5.94
- Van der Waals volume : 124
- Lipid solubility : LogP = 4.5
Biochemical significance
On the one hand, isoleucine is required as a building block for protein synthesis. On the other hand, it can also be used to generate energy in muscle cells . This plays a role in protein-rich food or during longer exertion and in phases of hunger when the body draws on its own reserves. The breakdown of isoleucine provides acetyl-CoA and propionyl-CoA .
Estimates of the daily requirement for healthy adults range, depending on the method used, from 7.5 to 28 mg isoleucine per kilogram of body weight. In the human organism, isoleucine occurs almost exclusively in a bound state. The concentration of free isoleucine in the blood is around 7 mg / l; 10 to 15 mg are excreted in the urine every day.
Extraction
The main extraction methods are fermentation processes in which glucose-containing solutions with the addition of L - threonine are converted by microorganisms producing L- isoleucine. In contrast, a mixture of the natural amino acids L- leucine and L- isoleucine is obtained by hydrolysis of proteins and subsequent separation operations of the hydrolysates . These constitutional isomers can then be z. B. separate by an enzymatic process.
use
As a component of amino acid infusion solutions for parenteral nutrition, L -Isoleucine, along with other amino acids, is widely used in human medicine. An orally administered “chemically defined diet” containing L- isoleucine was developed for patients with impaired digestion . In this diet, the amino acids are the source of nitrogen; all vital nutrients are in a chemically precisely defined form.
Web links
Individual evidence
- ↑ a b c d e f Entry on L-isoleucine in the GESTIS substance database of the IFA , accessed on February 5, 2018(JavaScript required) .
- ^ A b Hans Beyer, Wolfgang Walter: Textbook of Organic Chemistry . Hirzel Verlag, Stuttgart 1991, ISBN 3-7776-0485-2 , p. 823.
- ↑ S. Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois . ( Memento of the original from June 15, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF) Berlin 2015.
- ↑ F. Ehrlich: About the natural isomer of leucine . In: Ber Deutsche Chem Ges , Volume 37, pp. 1809-1840 (1904), doi: 10.1002 / cber.19040370295 .
- ↑ F. Ehrlich: About the natural isomer of leucine. Constitution and synthesis of isoleucine . In: Ber Deutsche Chem Ges , Volume 40 (2), pp. 2538-2562 (1907), doi: 10.1002 / cber.190704002181 .
- ↑ nutrient database of the US Department of Agriculture , 21st edition.
- ↑ Bernd Hoppe, Jürgen Martens: Amino acids - production and extraction . In: Chemistry in our time , 1984, 18, pp. 73-86.
- ↑ JM Berg, JL Tymoczko, L. Stryer: Biochemistry. 6th edition. Spectrum Academic Publishing House, Elsevier, Munich 2007; Pp. 735, 746; ISBN 978-3-8274-1800-5 .
- ↑ AV Kurpad, MM Regan, T. Raj, JV Gnanou: Branched-chain amino acid requirements in healthy adult human subjects . In: J. Nutr. , 2006, 136 (1 Suppl), pp. 256S-263S, PMID 16365094 .
- ^ Roche Lexicon Medicine. 5th edition. Urban & Fischer Verlag, Elsevier, Munich 2003, ISBN 978-3-437-15150-7 .
- ↑ a b Yoshiharu Izumi, Ichiro Chibata and Tamio Itoh: Production and Use of Amino Acids . In: Angewandte Chemie , 1987, 90, pp. 187-194. Angewandte Chemie International Edition in English , 1978, 17, pp. 176-183.
- ↑ Hitoshi Enei, Kenzo Yokozeki, Kunihiko Akashi: Recent Progress in Microbial Production of Amino Acids . Gordon & Breach Science Publishers, 1989, ISBN 978-2-88124-324-0 , p. 61.
- ↑ Jürgen Martens , Horst Weigel: Enzymatic Separation of L -Leucine and L -Isoleucine . In: Liebigs Annalen der Chemie , 1983, pp. 2052-2054.