Cysteine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structure of L- cysteine, the naturally occurring enantiomer | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Cysteine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 3 H 7 NO 2 S | |||||||||||||||||||||
| Brief description |
colorless solid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 121.16 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
220-228 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cysteine (pronounced: Cyste-ín), abbreviated to Cys or C , is an α - amino acid with the side chain –CH 2 –SH that contains sulfur . Only the naturally occurring enantiomeric form L - cysteine [synonym: ( R ) -cysteine] is a proteinogenic amino acid; in adults it can be formed in the liver from the sulfur-containing amino acid L - methionine .
By oxidation of the sulfhydryl groups , two cysteine residues can form a disulfide bridge with one another , which creates cystine . Such disulfide bridges stabilize the tertiary and quaternary structure of numerous proteins and are important for the formation and maintenance of functional conformations .
Isomerism
Cysteine can exist in the enantiomeric forms D and L , with only the L form occurring in proteins . Since sulfur is assigned a higher priority than oxygen according to the CIP nomenclature , L- cysteine - like the disulfide L - cystine and L - selenocysteine - is a proteinogenic amino acid with an ( R ) configuration .
In this article, the information on physiology concerns L- cysteine alone . If cysteine is spoken of without any addition , L- cysteine is generally meant. The racemic DL- cysteine [synonym: ( RS ) -cysteine] and enantiomerically pure D- cysteine [synonym: ( S ) -cysteine] are synthetically accessible and are of little practical importance.
The racemization of L- amino acids can be used for amino acid dating - an age determination for fossil bone material.
| Enantiomers of cysteine | ||
| Surname | L- cysteine | D- cysteine |
| other names | ( R ) -cysteine | ( S ) -cysteine |
| Structural formula |  |
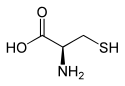
|
| CAS number | 52-90-4 | 921-01-7 |
| 3374-22-9 (racemate) | ||
| EC number | 200-158-2 | 213-062-0 |
| 222-160-2 (racemate) | ||
| ECHA info card | 100,000,145 | 100,011,875 |
| 100.020.147 (racemate) | ||
| PubChem | 5862 | 92851 |
| 594 (racemate) | ||
| DrugBank | DB00151 | DB03201 |
| - (racemate) | ||
| Wikidata | Q186474 | Q16633812 |
| Q27089394 (racemate) | ||
history
L- cysteine was first isolated from kidney stones as cystine in 1810 by the English scientist William Hyde Wollaston , from which the name ( ancient Greek κύστις küstis , German “bladder” , “ urinary bladder ”) is derived. Wollaston initially called the new substance "cystic oxide" before Jöns Jakob Berzelius later named it cystine. The Swedish chemist Count Mörner succeeded in isolating it from proteins for the first time in 1899. Before that, the Freiburg professor Eugen Baumann had succeeded for the first time in obtaining the actual amino acid cysteine by reducing cystine. Emil Fischer was finally able to explain the structural formula of cysteine beyond any doubt.
Occurrence
L- cysteine is found in proteins, but not all proteins contain cysteine. Computational analysis of 207 unrelated proteins resulted in an average mass fraction of 2.6% cysteine; in the same analysis, 1.7% cysteine was determined for whey protein .
High L- cysteine content (and thus high stability) can be found e.g. B. in keratin : feather keratin contains about 7%, wool keratin 11 to 17% cysteine. But also very small sterically stabilized proteins such as snake toxins ( myotoxin , neurotoxin etc .; about 40 to 70 amino acids) contain 10 to 14% cysteine in the form of cystine (disulfide bridges).
Food
The following examples give an overview of the cysteine content and each relate to 100 g of the food; the percentage of cysteine in the total protein is also given.
| Food | Total protein | Cysteine | proportion of |
|---|---|---|---|
| Pork, raw | 20.95 g | 242 mg | 1.2% |
| Chicken breast fillet, raw | 21.23 g | 222 mg | 1.0% |
| Salmon, raw | 20.42 g | 219 mg | 1.1% |
| Chicken egg | 12.57 g | 272 mg | 2.2% |
| Cow's milk, 3.7% fat | 3.28 g | 30 mg | 0.9% |
| Sunflower seeds | 20.78 g | 451 mg | 2.2% |
| Walnuts | 15.23 g | 208 mg | 1.4% |
| Wholemeal wheat flour | 13.70 g | 317 mg | 2.3% |
| Wholemeal corn flour | 6.93 g | 125 mg | 1.8% |
| Rice, unpeeled | 7.94 g | 96 mg | 1.2% |
| Soybeans, dried | 36.49 g | 655 mg | 1.8% |
| Peas, dried | 24.55 g | 373 mg | 1.5% |
Cysteine is one of the non-essential amino acids. At least for adults, it is certain that the body can synthesize all of its cysteine requirements from the essential amino acid methionine , provided that the diet contains enough of it. Whether cysteine in turn is able to replace part of the methionine is still the subject of research. Sometimes cysteine and methionine are grouped under the term sulfur-containing amino acids and a common requirement is stated. It should be noted that this is not a real combined requirement, but only the methionine requirement for a cysteine-free diet.
The terms cysteine and cystine are often used synonymously in the literature and nutrient databases when specifying the cysteine content. Strictly speaking, this is not correct, as cysteine denotes the monomer and cystine denotes the dimer formed by a sulfur bridge. However, many common analytical methods do not quantify the two compounds separately.
Biochemical significance
Various functions of cysteine in the organism are derived from the relative reactivity of its free thiol group . During protein folding, a disulfide bridge (–S – S–) can form between cysteine residues of the same polypeptide chain , which come into spatial proximity to one another during the folding process. The bridging by an additional covalent bond between amino acids at non-adjacent positions in the chain increases the stability of their spatial arrangement, the tertiary structure . In the folding protein molecule, under the action of protein disulfide isomerases, a disulfide bridge, also known as a cystine bridge, can possibly be shifted to other cysteine residues. The cross- bridges formed via the S atom of cysteine residues play an essential role in the folding of the native protein and stabilize its tertiary structure through covalent bonds. Stabilizing disulfide bridges occur in many, mainly secretory and extracellular proteins, for example in insulin . They can also stabilize the quaternary structure of a protein complex made up of several polypeptide chains or link chains with one another, for example in the case of antibodies , and link linked chains to form bundles, as in the case of keratins . In the case of short peptides with cysteine at the chain end, such as the nonapeptide oxytocin , a proteohormone , the formation of a disulfide bridge creates a ring-shaped structure, which makes unspecific degradation by peptidases difficult.
A larger group of enzymes has iron-sulfur clusters coordinated by cysteine residues . The relatively reactive thiol group of cysteine can also be directly involved in the catalytic mechanism, as in glyceraldehyde-3-phosphate dehydrogenase , where cysteine binds the substrate to the active site.
Cysteine is also a starting material in the biosynthesis of compounds such as glutathione , coenzyme A and taurine .
properties
Cysteine is mainly present as an "inner salt" or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value), at which the cysteine also has its lowest solubility in water. The isoelectric point of cysteine is at pH 5.02.
Cysteine could be counted among the non- essential amino acids as it can be produced by the body. However, the essential amino acid methionine is required for this. Therefore, cysteine is usually considered to be semi-essential. As a component of many proteins and enzymes , it is often involved in the catalytic mechanism.
In a neutral to alkaline aqueous solution, when air is admitted, oxidation to cystine takes place . When exposed to stronger oxidizing agents, cysteic acid is formed.
Technical extraction
Like almost all other amino acids , L- cystine can be obtained by hydrolysis through the action of hydrochloric acid on proteins such as keratin (mostly from keratin-rich tissues such as human or animal hair or feathers) . The L- cystine obtained in this way can then be converted into L- cysteine by electrochemical reduction .
Since today there is a clear trend away from animal products to plant-based alternatives among consumers, cysteine, which was obtained from plants, is now also being used. This is made by fermentation from raw materials on a vegan basis and inorganic trace elements. The L -cysteine is used in the baking industry as Teigweichmacher.
For some time now, the representation has also been made by fermentation with bacteria , e.g. B. Escherichia coli , also possible using genetically modified organisms (see illustration of tryptophan ).
Racemic cysteine ( DL- cysteine) can be obtained fully synthetically from 2-chloroacetaldehyde , sodium hydrogen sulfide , ammonia and acetone via the intermediate product 2,2-dimethyl-3-thiazoline obtained from the Asinger reaction . Then, hydrogen cyanide annealed and acid hydrolysis.
Biosynthesis and metabolism
Cysteine is biosynthetically formed in the liver from serine , which provides the basic structure, and methionine via homocysteine , which contributes the SH group . The enzymes cystathionine synthetase and cystathionase are required for this. Serine or methionine deficiencies consequently inhibit cysteine synthesis.
The amino acid can be broken down by α, β - elimination . This creates amino acrylate and hydrogen sulfide (H 2 S). H 2 S is oxidized to sulfate (SO 4 2− ). Amino acrylate isomerizes to iminopropionate , which hydrolytically splits off its amino group and thus becomes pyruvate .
It can also become β-mercaptopyruvate through transamination . The sulfite transferase transfers sulfite to the thiol group, thereby converting it into a thiosulfate . After hydrolysis of the carbon-sulfur bond, pyruvate is then released; the thiosulfate (S 2 O 3 2− ) is oxidized to the sulfate. Cysteine can also be oxidized at the SH group and then decarboxylated to taurine .
By genetic defects in Cystintransporter tract stomach and a resumption, after recording in the kidney in cystinuria arise. The mutation in the rBAT gene also affects the metabolism of the amino acids lysine , arginine and ornithine , i.e. the polyamino-amino acids.
Therapeutic functions
Active pharmaceutical ingredients are produced from L- cysteine on an industrial scale, e.g. B. ( R ) - S - carboxymethylcysteine and ( R ) - N - acetylcysteine (ACC and NAC, respectively). These two active pharmaceutical ingredients are used as oral mucolytic agents to liquefy the often viscous bronchial mucus in chronic bronchitis and chronic obstructive pulmonary disease. When cysteine is given, the increased bronchial mucus that forms in the course of these diseases becomes thinner and can therefore be coughed up more easily. Cysteine also increases a number of lymphocyte functions, such as cytotoxic T cell activity. Cysteine and glutathione prevent the expression of NF-AT , the nuclear transcription factor, in stimulated T-cell lines. In vitro studies show that the stimulating effect of TNF ( tumor necrosis factor ), induced by free radicals, on HIV replication in monocytes can be inhibited by sulfur-containing antioxidants. These fundamental studies suggest that treating inflammatory diseases and AIDS with cysteine could potentially be useful with it.
Cysteine can complex heavy metal ions . It is therefore used, among other things, as a therapeutic agent for silver poisoning. Since it binds free radicals to the thiol group , cysteine is also used to prevent radiation damage. In fetuses, premature babies and newborns, as well as in liver cirrhosis , the activity of the enzyme cystathionase is either absent or severely restricted. In these cases, an exogenous supply of cysteine is necessary. It is a radical scavenger that renders cell-damaging substances harmless and for which recent studies postulate a certain preventive function against neurodegenerative diseases .
In the very rare neurodegeneration with brain iron accumulation causes a mutation in the enzyme pantothenate kinase encoding PANK2 gene that there will be an accumulation of cysteine iron - complexes in the brain - especially in the globus pallidus and substantia nigra pars reticulata is -. This in turn leads to an increase in free radicals and ultimately to oxidative damage to the nerve cells of the brain.
Cysteine is a component of amino acid infusion solutions for parenteral nutrition.
Food additive
L- cysteine is used in the form of the hydrochloride as a flour treatment agent and baking agent in the manufacture of baked goods . It softens the glue by depolymerizing the molecules of the glutenin fraction through thiol-disulfide exchange with the intermolecular disulfide bonds ( i.e. breaking the bonds that hold the long chain molecules together). As a result, the dough becomes more elastic and develops faster. With high-gluten flour , a higher volume can be achieved because the propellant gas (such as the carbon dioxide formed by the yeast ) can loosen the dough more easily. Cysteine hydrochloride can also be added to the production of pasta in order to speed up dough production (an addition of 0.01% reduces the mixing or kneading time by 15–20%). It inhibits the formation of melanoidins in non-enzymatic tanning and thus counteracts discoloration. In addition to these uses, like other amino acids, cysteine acts as a flavor , flavor enhancer, and nutrient .
L- cysteine hydrochloride or hydrochloride monohydrate is approved under European food law as an additive without maximum quantity restrictions ( quantum satis ) under the number E 920 . In principle, it must be declared, but not if it is no longer technologically effective in the labeled product in accordance with Article 20 of the Food Information Ordinance and Section 9, Paragraph 8, No. 1 of the Additive Authorization Ordinance . In the opinion of the Backmittelinstitut (an institution of the Association of Baking Ingredients ), the technological effectiveness of cysteine, which is used as a flour treatment agent (i.e. was added to the flour before doughing), does not extend to the finished baked goods, but to the dough , if it is used as a Semi-finished product is offered. As a result, it does not need to be labeled for finished baked goods. The Federation of German Consumer Organizations does not share this legal opinion.
Further areas of application
In Japanese hairdressing salons, cysteine, which is able to break disulfide bonds in keratin, replaces the strong smelling thioglycolic acid common in Europe when it comes to preparing hair for permanent waves . Cysteine is also used in other cosmetic products.
Web links
Individual evidence
- ↑ Entry on E 920: L-cysteine in the European database for food additives, accessed on August 11, 2020.
- ↑ Entry on CYSTEINE in the CosIng database of the EU Commission, accessed on August 11, 2020.
- ↑ a b c d e f data sheet (R) - (+) - cysteine (PDF) from Merck , accessed on March 23, 2011.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of Amino Acids, pp. 7-1.
- ↑ a b c Entry on L-cysteine. In: Römpp Online . Georg Thieme Verlag, accessed on July 21, 2011. .
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, 62, 1982, ISBN 3-527-25892-2 .
- ^ William Hyde Wollaston: On Cystic Oxide, a New Species of Urinary Calculus . In: Phil. Trans. Royal. Soc. tape 100 , 1810, pp. 223 ff . (English).
- ↑ Sabine Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. Berlin 2015.
- ↑ Eugen Baumann: About cystine and cysteine . In: Journal of Physiological Chemistry . tape 8 , no. 4 , 1884, p. 299 ff .
- ^ Emil Fischer, K. Raske: Conversion of the l-serine into active natural cystine . In: Reports of the German Chemical Society . tape 41 , no. 1 , 1908, p. 893 ff .
- ↑ Abby Thompson, Mike Boland, Harjinder Singh: Milk Proteins: From Expression to Food . Academic Press, 2009, ISBN 978-0-08-092068-9 , pp. 492 (English, limited preview in Google Book search).
- ↑ David Plackett: Biopolymers: New Materials for Sustainable Films and Coatings . Wiley, 2011, ISBN 978-1-119-99432-9 , pp. 115 (English, limited preview in Google Book search).
- ↑ nutrient database of the US Department of Agriculture , 22nd edition.
- ↑ Ronald O. Ball, Glenda Courtney-Martin, Paul B. Pencharz: The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans . In: The Journal of Nutrition . tape 136 , 6 Suppl, June 2006, ISSN 0022-3166 , p. 1682S-1693S , PMID 16702340 (English).
- ↑ M. Aristoy, F. Toldra: Amino Acids . In: LML Nollet (Ed.): Handbook of Food Analysis . 2nd Edition. Marcel Dekker AG, New York / Basel 2004, ISBN 0-8247-5036-5 , p. 95, 110 (English).
- ↑ Peter Heinrich, Matthias Müller, Lutz Graeve (eds.): Löffler / Petrides Biochemistry and Pathobiochemistry. 9th edition, Springer-Verlag, Heidelberg 2014, ISBN 978-3-642-17971-6 , p. 485.
- ↑ JM Berg, JL Tymoczko, L. Stryer: Biochemistry. 6th edition. Spektrum Akademischer Verlag, Elsevier GmbH, Munich 2007, ISBN 978-3-8274-1800-5 , pp. 38f, 48, 494f, 570.
- ^ D. Doenecke, J. Koolman, G. Fuchs, W. Gerok: Karlsons Biochemie und Pathobiochemie . Ed .: Peter Karlson, Detlef Doenecke. 15th, completely revised. and redesigned edition. Thieme, Stuttgart 2005, ISBN 3-13-357815-4 , p. 41, 208, 219 .
- ↑ PM Hardy: The Protein Amino Acids . In: GC Barrett (Ed.): Chemistry and biochemistry of the amino acids . Chapman and Hall, London / New York 1985, ISBN 0-412-23410-6 , pp. 9 (English).
- ↑ a b Plant-Based L-Cysteine for Dough Softening. wacker.com, accessed on August 8, 2020 .
- ↑ Jürgen Martens , Heribert Offermanns , Paul Scherberich: A simple synthesis of racemic cysteine. In: Angewandte Chemie . Volume 93, 1981, p. 680, doi: 10.1002 / anie.19810930808 ; Angewandte Chemie International Edition. English, Volume 20, 1981, p. 668, doi: 10.1002 / anie.198106681 .
- ↑ P. Fürst, H.-K. Biesalki among others: nutritional medicine . Ed .: Hans-Konrad Biesalski, Olaf Adam. 3rd, exp. Edition. Thieme, Stuttgart 2004, ISBN 3-13-100293-X , p. 94 .
- ↑ B. Zhou, SK Westaway, B. Levinson, MA Johnson, J. Gitschier, SJ Hayflick: A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome . In: Nature Genetics . tape 28 , no. 4 , August 2001, ISSN 1061-4036 , p. 345-349 , doi : 10.1038 / ng572 , PMID 11479594 (English).
- ^ Siegfried Ebel, Hermann J. Roth (Ed.): Lexicon of Pharmacy . Thieme, Stuttgart 1987, ISBN 3-13-672201-9 , p. 66 .
- ↑ a b Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Textbook of food chemistry . 6th completely revised edition. Springer, Berlin / Heidelberg 2008, ISBN 978-3-540-73201-3 , doi : 10.1007 / 978-3-540-73202-0 .
- ^ Peter Kuhnert: Lexicon of food additives . 4th, completely revised edition. Behr, Hamburg 2014, ISBN 978-3-95468-118-1 .
- ↑ Regulation (EC) No. 1333/2008 in the consolidated version of February 9, 2016
- ↑ Regulation (EU) No. 231/2012 in the consolidated version of October 20, 2015
- ↑ Martina Bröcker, Amin Werner: The technological effectiveness of food additives in bread, small baked goods, fine baked goods and dough pieces . In: bmi aktuell . December 2007 ( wissensforum-backwaren.de [PDF; 134 kB ]). The technological effectiveness of food additives in bread, biscuits, fine baked goods and dough pieces ( Memento of the original from April 6, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Flour treatment agents. German Consumer Association V., accessed April 7, 2016 .


