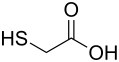
Mercaptoacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Mercaptoacetic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 4 O 2 S | ||||||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 92.12 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.325 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−10 ° C |
||||||||||||||||||
| boiling point |
220 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| pK s value |
3.68 (25 ° C) |
||||||||||||||||||
| solubility |
completely miscible with water, ethanol , chloroform , toluene and diethyl ether |
||||||||||||||||||
| Refractive index |
1.5080 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 1 ml m −3 or 4 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Mercaptoacetic acid (other name: thioglycolic acid) is a colorless, viscous liquid and belongs to the group of thiols , its salts are called thioglycolates. It is chemically related to glycolic acid .
synthesis
The synthesis takes place from chloroacetic acid and sodium hydrogen sulfide by nucleophilic substitution .
use
It has been used in depilatories and hairdressing products for permanent waves since around 1940 . These contain about 1 to 11% mercaptoacetic acid as ammonium salt ( ammonium thioglycolate ):
The use for permanent waves is based on the interaction between keratin - which hair is made of - and thioglycolic acid, discovered by Leonor Michaelis . In this case, be disulfide bridges between two cysteine of the keratin by units reduction separately.
Potassium thioglycolate may also be found in depilatories. It is also used in leather processing and in the manufacture of stabilizers for PVC . Furthermore, it can be used to detect iron ions through an intense red color. The reaction of mercaptoacetic acid with cyclic imines leads to bicyclic lactams.
safety instructions
When heated, mercaptoacetic acid decomposes with the formation of toxic fumes ( hydrogen sulfide and sulfur oxides ).
Individual evidence
- ↑ entry to THIOGLYCOLIC ACID in CosIng database of the European Commission, accessed on 28 December of 2019.
- ↑ a b c Entry on sulfanylacetic acid. In: Römpp Online . Georg Thieme Verlag, accessed on March 27, 2014.
- ↑ a b c d e f g h i Entry on thioglycolic acid in the GESTIS substance database of the IFA , accessed on April 17, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-42.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-484.
- ↑ Entry on Mercaptoacetic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 68-11-1 or mercaptoacetic acid ), accessed on November 2, 2015.
- ^ Siegfried Hauptmann: Organic Chemistry , Verlag Harry Deutsch, 1985, page 476, ISBN 3-87144-902-4 .
- ^ Leonor Michaelis. 1875–1949 (PDF; 2.4 MB). A Biographical Memoir by L. Michaelis, DA MacInnes, S. Granick J., National Academy of Sciences.
- ^ W. Bergfeld, D. Belsito, J. Marks, Jr., F. Andersen: Safety of ingredients used in cosmetics , In: Journal of the American Academy of Dermatology . 2005; 52: 125-132.
- ↑ S. Köpper, K. Lindner, J. Martens : The addition of mercaptocarboxylic acids to 3-thiazolines and subsequent lactamization , Tetrahedron 48 (1992) 10277-10292.


