Keratins
Keratin (from Greek κέρας kéras "horn", genitive kératos ) is a collective term for various water-insoluble fiber proteins that are formed by animals and characterize the horny substance . A distinction is made between α- and β-keratins according to their molecular conformation as an α-helix or β-sheet .
Keratins are the major constituent of mammalian hair , finger and toe nails , claws , jaws , hooves , horns , nose horns of the rhino , spines of the hedgehog , Barten of whales , beaks , and feathers of birds , horny scales and outer armor covering of reptiles .
Structure and properties
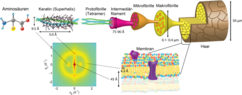
Their fiber structure increases the strength of the keratins: the individual amino acid chains form a right-handed α-helix . Two of these helices combine to form a left-handed superhelix and two of these superhelices in α-keratin to form a protofibril. Several protofibrils combine to form a microfibril, these bundle together and form macrofibrils. The fibers are stiffer, the stronger their components are cross-linked by disulfide bridges of the amino acid L - cysteine . The keratin in horns and claws contains more disulfide bridges than that in wool and hair. Leonor Michaelis discovered that disulfide bridges are reduced by thioglycolic acid . This was the biochemical basis for the perm .
Before cornification and generally in epithelia of vertebrates and other animal groups, α-keratins (or cytokeratins) are in the form of loosely organized keratin filaments. These belong to the intermediate filaments which, together with microtubules and microfilaments, form the cytoskeleton of the eukaryotic cells. There are currently 20 known cytokeratin proteins (see table) with a molecular mass between 40 and 68 kDa. KRT1 to KRT8 belong to the neutral-basic type A subfamily, KRT9 to KRT20 to the acidic type B subfamily. In pairs, these form a heterodimer complex in the intermediate filaments of a type A and a type B cytokeratin. The distribution patterns of these complexes differ considerably in different epithelial cells, so that the origin of these cytokeratins can be narrowed down with an antibody detection against the subtypes KRT1 to KRT20. This is used in medicine in pathological diagnostics to determine the origin of tumor metastases . Mutations in various keratin genes are responsible for a number of rare hereditary diseases ( ichthyosis ).
| A (neutral- basic) |
B (angry) |
Occurrence | pathology |
|---|---|---|---|
| KRT1 , KRT2 | KRT9 , KRT10 | multilayered keratinized epithelium ( epidermis ) | Ichthyosis hystrix Curth-Macklin ; Bullous Congenital ichthyosiform Erythroderma Brocq (EHK); keratosis palmoplantaris striata III; other ichthyoses (BCIE; AEI; CRIE) |
| KRT3 | KRT12 | Cornea ( cornea ) | Corneal dystrophy |
| KRT4 | KRT13 | multilayered, uncornified epithelium | Naevus spongiosus albus |
| KRT5 | KRT14 , KRT15 | Basal cells of complex epithelia as well as myoepithelial cells | Epidermolysis bullosa simplex; Dowling Degos Disease ; Naegeli syndrome |
| KRT6A | KRT16 , KRT17 | multi-layered uncornified squamous epithelium, proliferation | Pachyonychia congenita ; div. nevus, keratoderma |
| KRT7 | KRT19 | monolayer epithelium, luminal gland cells | |
| KRT8 | KRT18 , KRT20 | monolayer epithelium, luminal gland cells |
An example of a β-keratin is the fibroin or silk protein found in cobwebs and silk . In contrast to the α- or cytokeratins, it is not an intracellular structural protein, but a product excreted by the spinal glands . Because of its sheet structure, it is much less ductile than the helically built α-keratins.

Technical use
From keratin-containing natural products are L cysteine, L - tyrosine and other proteinogenic amino acids on an industrial scale produced. To do this, the natural substances are first hydrolyzed with hydrochloric acid. The hydrolyzate is neutralized with ammonia . The L -amino acids are then separated off due to their different solubilities or isolated according to the principle of ion exchange chromatography .
Keratin products (e.g. sheep's wool fleece) are used to break down formaldehyde . In long-term research work , the German Wool Research Institute at RWTH Aachen University together with the eco Institute in Cologne has proven that such products are able to remove formaldehyde from the room air. Kindergartens, schools and private houses (many prefabricated houses from the 1970s and 1980s) contaminated with formaldehyde have already been renovated in this way in recent years.
Web links
Individual evidence
- ↑ Bernd Hoppe, Jürgen Martens : Amino acids - production and extraction. In: Chemistry in Our Time . 1984, 18, pp. 73-86.
- ↑ Sheep wool insulation materials. ( Memento of the original from February 18, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. wecobis.de
- ↑ Robert Sweredjuk, Gabriele Wortmann, Gerd Zwiener, Fritz Doppelmayer: Sheep wool as a reactive sorbent for air pollutants in the interior. (PDF; 201 kB).
- ↑ G. Wortmann, G. Zwiener, R. Sweredjiuk, F. Doppelmayer, FJ Wortmann, 1999 Sorption of indoor air pollutants by sheep's wool: Formaldehyde as an example. Proceedings of the International Wool Textile Organization, Report CTF 3, Florence, Italy, June 1999.