α-helix
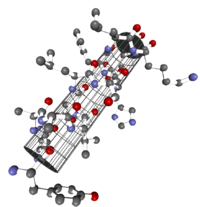
In biochemistry, the α- helix is a common expression of the secondary structure of a protein . It belongs to the most stable natural conformations of an amino acid sequence and is almost omnipresent in the secondary structure.
The secondary structure of a protein is understood to mean the spatial structure of the amino acid chain without taking the side groups into account. The secondary structure of a protein is derived from its primary structure (amino acid sequence). Superordinate structural levels are the tertiary structure and the quaternary structure . The three-dimensional structure of a protein is crucial for its selective function (see protein structure ).
history
In the late 1930s, William Astbury began to perform crystal structure analyzes on crystalline peptides . It was found that certain spatial features repeat regularly, where hydrogen bonds within the molecule were suspected. However, he was not yet aware of the planarity of the peptide bond . The most common spatial structures were later called α-helix and β-sheet . Linus Pauling , Robert Brainard Corey and Herman Branson proposed a model of the α-helix in 1951. The α in "α-helix" does not contain any scientific statement, but only expresses the fact that the α-helix was found in front of the β-sheet. The Ramachandran plot developed by G. N. Ramachandran allowed them to be identified based on the dihedral angles of the consecutive amino acids in the protein , similar to the Janin plot developed later .
structure
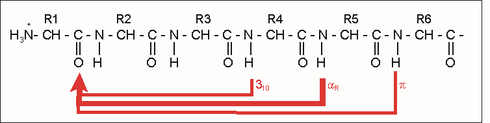
The α-helix is a right-handed spiral (preferably of L-amino acids) with an average of 3.6 amino acid side chains per revolution. A length of p = 0.54 nm (5.4 Å) is achieved per turn. This advance is known as the pitch. It is the product of the shift (also called translation) (0.15 nm) and remainders per turn (3.6). This distance between residues is the reason that amino acids that are three or four places apart in the primary structure are in close proximity in the helix structure. The α-helix is stabilized by a hydrogen bond between the carbonyl oxygen of the nth and the amide proton of the (n + 3) th amino acid of the same molecule.
The CO and NH groups must be close to one another in order to form the hydrogen bond. The narrowest configuration is provided by a coiled strand in which the two groups lie on top of each other. The side chains point outwards. The amino acid proline (“structure breaker”) cannot be easily inserted into the helix (this is only possible at positions 1–4, seen from the amino end). As a result, there are deviations from the regular structure at points where proline occurs. α-Helices are very stable and, as rigid cylinders, can form a kind of skeleton of the protein. Therefore, they are often not depicted as helices in protein structures but as cylinders. A protein with a predominantly helical structure is myoglobin , a muscle protein related to hemoglobin .
An α-helix is often only stable in the context of a protein, which is why additional stabilizing bonds are often introduced into isolated α-helices, e.g. B. by replacing the hydrogen bond with a CC bond, by cross-linking the amino acid side chains or by forming disulfide bridges .
Geometry of the helix and helix-helix interactions


Part A shows a “coiled coil” consisting of two, part B one consisting of three α-helices (projection). In addition, “tetrahelix bundle” structures were described.

α-Helices are the basis of typical fiber proteins (α-keratin, the basic substance of hair, myosin, a component of muscle fibers, etc.) but also, as introduced in the example of myoglobin, structure-giving components of soluble, globular proteins. In general, individual helices cannot take on this task, but ordered aggregates of two, three, four or more individual helices can.
The assembly to form such a "superhelix" is based on hydrophobic interactions in amphipathic helices. These are helices, one side of which is hydrophilic (facing the water) and the other side is hydrophobic and therefore capable of interactions. The structure of the helix means that the “hydrophobic band” does not run parallel to the helix axis, but surrounds the helix in the form of a stretched, left-handed spiral. When the hydrophobic bands of two or more helices approach each other, the superhelix known as a “ coiled coil ” is formed.
Helix prediction
The first efforts to predict secondary protein structures go back to the 1960s and have been continuously refined with the advent of modern X-ray structure analysis. A very helpful, rational approach to predicting the α-helix is associated with the name Marianne Schiffer and follows on from the above considerations. According to the n +/- 3.4 criterion, a remainder n can pair with residues that are three or four positions away. Are z. B. residues 1, 4 and 5 hydrophobic, they can interact and thus stabilize a helix structure. The same applies to residues 6, 3 and 2 etc. This prediction scheme initially showed its value for insulin and myoglobin.
With the publication of further X-ray structure analyzes, the “helical wheel” approach increasingly gave way to statistical methods. An early approach of this kind goes back to Chou and Fasman (1974, 1978).
The table below shows the helical potentials (Pα) of amino acid residues. Pα corresponds to the relative frequency with which the amino acid is represented in the helix. With a Pα well above 1, an amino acid is referred to as a "helix former", with a Pα well below 1 as a "helix breaker".
| amino acid | Pα |
|---|---|
| Glu | 1.59 |
| Ala | 1.41 |
| Leu | 1.34 |
| Mead | 1.30 |
| Gln | 1.27 |
| Lys | 1.23 |
| Arg | 1.21 |
| Phe | 1.16 |
| Ile | 1.09 |
| His | 1.05 |
| Trp | 1.02 |
| Asp | 0.99 |
| Val | 0.90 |
| Thr | 0.76 |
| Asn | 0.76 |
| Cys | 0.66 |
| Tyr | 0.61 |
| Ser | 0.57 |
| Gly | 0.43 |
| Per | 0.34 |
Summary of the helix parameters
| A - Relation of the remnants to each other | |||
| n + 4 | Remnants 1 and 5 | H-bridge | -C = O ··· HN- |
| n +/- 3, 4 | Remnants 1 and 4 or 5 | same side | “Hydrophobic arc”, α potential |
| n + 18 | Remnants 1 and 19 | ecliptic | 5 x 3.6 = 18; "Repeat unit" |
| n + 7 | Remnants 1 and 8 | "Almost ecliptic" | 2 x 3.6; "Heptad repeat" |
| B - physical parameters | |||
| n = 3.6 | Remnants per turn | ||
| d = 1.5 A | axial shift per remainder | ||
| p = 5.4 Å = nxd | "Pitch" (distance between the turns) | ||
| a = 100 ° = 360 ° / n | Angle (sector) per amino acid | ||
Other forms of secondary structure

In addition to the α-helix and the β-sheet, there are other types of secondary structure (secondary structure motifs). Other common motifs are:
The parts of the primary structure of a protein that do not belong to a motif are called random loops ( random coil structures ). These structures are also significantly involved in the formation of the entire protein structure .
literature
- M. Schiffer, AB Edmundson: Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. In: Biophysical Journal. 7, 1967, pp. 121-135.
- PY Chou, GD Fasman: Empirical predictions of protein conformation. In: Annual Review of Biochemistry . 47, 1978, pp. 251-276.
- C. Cohen, DAD Parry: α-Helical coiled-coils - a widespread motif in proteins. In: Trends in biochemical sciences . 11, 1986, pp. 245-248.
- S. Kamtekar, JM Schiffer, H. Xiong, JM Babik, MH Hechtr: Protein design by binary patterning of polar and nonpolar amino acids. In: Science . 262, 1993, pp. 1680-1685.
Web links
Individual evidence
- ^ William T. Astbury, S. Dickinson, K. Bailey: The X-ray interpretation of denaturation and the structure of the seed globulins. In: The Biochemical journal. Volume 29, Number 10, October 1935, pp. 2351-2360.1, PMID 16745914 . PMC 1266766 (free full text).
- ^ William T. Astbury: The structural proteins of the cell. In: The Biochemical journal. Volume 39, Number 5, 1945, p. Lvi, PMID 21020817 .
- ^ WT Astbury, R. Reed, LC Spark: An X-ray and electron microscope study of tropomyosin. In: The Biochemical journal. Volume 43, Number 2, 1948, pp. 282-287, PMID 16748402 . PMC 1274681 (free full text).
- ^ Linus Pauling, Robert Brainard Corey, Herman R. Branson: The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. In: Proceedings of the National Academy of Sciences . Volume 37, Number 4, April 1951, pp. 205-211, PMID 14816373 . PMC 1063337 (free full text).
- ↑ JM Scholtz, RL Baldwin: The mechanism of alpha-helix formation by peptides. In: Annual review of biophysics and biomolecular structure. Volume 21, 1992, pp. 95-118, doi: 10.1146 / annurev.bb.21.060192.000523 . PMID 1525475 . rbaldwin.stanford.edu (PDF)
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry . 7th edition. Springer Spectrum, Heidelberg 2014, ISBN 978-3-8274-2988-9 , p. 40 .
- ↑ A. Winter, AP Higueruelo, M. Marsh, A. Sigurdardottir, WR Pitt, TL Blundell: Biophysical and computational fragment-based approaches to targeting protein-protein interactions: applications in structure-guided drug discovery. In: Quarterly reviews of biophysics. Volume 45, Number 4, November 2012, pp. 383-426, doi: 10.1017 / S0033583512000108 . PMID 22971516 .
- ↑ LK Henchey, AL Jochim, PS Arora: Contemporary strategies for the stabilization of peptides in the alpha-helical conformation. In: Current opinion in chemical biology. Volume 12, Number 6, December 2008, pp. 692-697, doi: 10.1016 / j.cbpa.2008.08.019 . PMID 18793750 . PMC 2650020 (free full text).
- ↑ RJ Platt, TS Han, BR Green, MD Smith, J. Skalicky, P. Gruszczynski, HS White, B. Olivera, G. Bulaj, J. Gajewiak: Stapling mimics noncovalent interactions of γ-carboxyglutamates in conantokins, peptidic antagonists of N-methyl-D-aspartic acid receptors. In: The Journal of biological chemistry. Volume 287, Number 24, June 2012, pp. 20727-20736, doi: 10.1074 / jbc.M112.350462 . PMID 22518838 . PMC 3370255 (free full text).
- ↑ P. Barthe, S. Rochette, C. Vita, C. Roumestand: Synthesis and NMR solution structure of an alpha-helical hairpin stapled with two disulfide bridges. In: Protein science: a publication of the Protein Society. Volume 9, Number 5, May 2000, pp. 942-955, doi: 10.1110 / ps.9.5.942 . PMID 10850804 . PMC 2144636 (free full text).
- ↑ Jeremy M. Berg: Stryer Biochemistry. 6th edition. Spektrum Akademischer Verlag, Heidelberg 2007, p. 56.
- ^ PDB Community Focus: Julian Voss-Andreae, Protein Sculptor. In: Protein Data Bank Newsletter. 32, winter, 2007, wwpdb.org (PDF)
- ↑ L. Moran, RA Horton, G. Scrimgeour, M. Perry: Principles of Biochemistry . Pearson, Boston MA 2011, ISBN 978-0-321-70733-8 , pp. 127 .