Myoglobin
| Myoglobin | ||
|---|---|---|
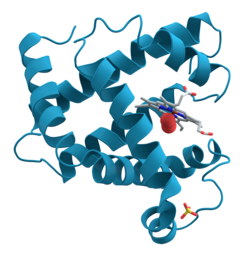
|
||
| Ribbon model of myoglobin (α-helices) with heme (rod model) | ||
| Properties of human protein | ||
| Mass / length primary structure | 153 amino acids , 17053 daltons | |
| Secondary to quaternary structure | Monomer | |
| Identifier | ||
| Gene names | MB ; MGC13548; PVALB | |
| External IDs | ||
| Occurrence | ||
| Homology family | Myoglobin | |
| Parent taxon | Chordates | |
Myoglobin is a muscle protein (from the Greek μυς, mys 'muscle' and Latin globus 'ball') from the group of globins , spherical proteins that contain an oxygen- binding heme group . Myoglobin can absorb and release oxygen and is responsible for the intramuscular transport of oxygen. It takes the oxygen from the blood from the hemoglobin and releases it again at the place of the physiological combustion processes in the muscle cells .
Scientific description
Myoglobin is a heme- based, globular , single-chain protein consisting of 153 amino acids with a molecular mass of 17.053 Daltons (17 kDa), which has the ability to reversibly bind oxygen (O 2 ) . The secondary structure of the protein consists of a total of eight α-helices .
Under physiological conditions it exists as a monomer . The active center of myoglobin is a heme b , i. H. a protoporphyrin IX with an iron (II) ion ligated via the four inner nitrogen atoms . The heme is bound to the protein matrix via a proximal histidine that is axially coordinated to the central iron ion . The second axial position is used to bind the oxygen molecule.
In contrast to the structurally related hemoglobin , myoglobin does not bind oxygen positively cooperatively . The binding behavior of O2 to myoglobin can be plotted on a coordinate system:
Ordinate degree of saturation Ys
The abscissa is the O2 partial pressure (pO2) in mbar
Ys = 0 means that no oxygen is bound and at Ys = 1 myoglobin is completely saturated with O2. The O2 pressure at half maximum saturation (Ys = 0.5) is referred to as p50.
If one examines the binding behavior of oxygen to myoglobin experimentally and plots the degree of saturation against the oxygen partial pressure, a hyperbolic curve results, which can be interpreted as follows: At low pO2, oxygen is almost completely absorbed by myoglobin. This phase corresponds to a steep and approximately linear increase in the curve. At higher pO2 it becomes increasingly difficult for the oxygen molecules to find "free" unsaturated myoglobin. The binding therefore approaches the state of full saturation. Represented by the increasingly weaker rising asymptotic part of the curve.
The occurrence of myoglobin is limited to cardiac and skeletal muscle cells of mammals . Here it is in high concentrations (up to about 100 µmol / l) and gives the muscle tissue its red color. The oxygen uptake can be easily followed by absorption spectroscopy , the characteristic Soret band of the heme shifts significantly from 418 to 434 nm when oxygen is absorbed.
Myoglobin is one of the globins . Closely related to myoglobin is cytoglobin , which was first described in 2002 and occurs in almost all cells of vertebrates. It probably also serves as an oxygen store in the cell. It even seems that myoglobin may have become the muscle-specific variant of cytoglobin through gene duplication during evolution .
meaning
The oxygen transport within the cell , from the cell membrane to the mitochondria , is regarded as a biological function . For this reason, its affinity for oxygen is also higher than with hemoglobin, which promotes the transport of oxygen towards the inside of the cell. The regulation of oxygen affinity observed in hemoglobin is also lacking in myoglobin. At least in the case of marine mammals , oxygen storage is also being discussed: whales have an approximately 5 to 10 times higher myoglobin content in their muscles than land mammals . In humans, the muscles contain around 6 grams of myoglobin per kilogram, in seals it is 52, in sperm whales even 56 grams. There it serves the marine mammals as an oxygen supply when diving.
The myoglobin of the sperm whale was therefore the first protein on which John Kendrew succeeded in determining the structure ( X-ray structure analysis ) in 1958 . This pioneering achievement was the basis for the later elucidation of the hemoglobin structure by Max Perutz (1959). Both scientists received the Nobel Prize in Chemistry in 1962 .
The iron content is a measure of the myoglobin content. In the case of pork, this has fallen by around 75% to an average of 4.1 mg / kg fresh mass within 30 years.
Importance in medicine
An increase in the myoglobin concentration in the blood serum of mammals indicates rhabdomyolysis and can be used as an indicator of a heart attack . Since an increased myoglobin value is unspecific, cardiac troponins are used today for heart diagnostics. In addition to the death of cardiac muscle cells, increased myoglobin values are also found in cases of damage to the skeletal muscles (extreme sport, epileptic seizures , multiple trauma , intramuscular injections , alcohol intoxication , muscle diseases, spills). If muscle tissue is injured during an electric shock, this results in the release of myoglobin. Strongly increased myoglobin concentrations can lead to acute kidney failure ( crush kidney ).
The plasma half-life of myoglobin is only 10 to 20 minutes because it is quickly excreted by the kidneys (glomerular filtration). The myoglobin level rises in a heart attack after approx. 1–2 hours and reaches its maximum after 4–6 hours. It falls back to the normal range within 12–24 hours.
Normal values in human blood : women up to 35 µg / l, men up to 55 µg / l
Normal value in human urine : women and men up to 0.3 mg / l
literature
- Brunori M: Myoglobin strikes back . In: Protein Sci. . 19, No. 2, February 2010, pp. 195-201. doi : 10.1002 / pro.300 . PMID 19953516 .
- Springer-Verlag GmbH: Biochemistry: An Introduction for Physicians and Natural Scientists . 3rd, corrected edition. Berlin, ISBN 978-3-662-54850-9 .
Web links
- Hemoglobin-Myoglobin in English.
- Myoglobin
Individual evidence
- ↑ Werner Müller-Esterl: Biochemistry an introduction for doctors and natural scientists . Ed .: Werner Müller-Esterl. 3rd edition, 2018, doi : 10.1007 / 978-3-662-54851-6 .
- ↑ Trent JT, Hargrove MS: A ubiquitously expressed human hexacoordinate hemoglobin . In: J. Biol. Chem. . 277, No. 22, May 2002, pp. 19538-45. doi : 10.1074 / jbc.M201934200 . PMID 11893755 .
- ↑ Frank Patalong : Evolution does repeat itself. Der Spiegel , April 18, 2015, accessed on August 18, 2015 .
- ↑ KENDREW JC, BODO G, DINTZIS HM, PARRISH RG, WYCKOFF H, PHILLIPS DC: A three-dimensional model of the myoglobin molecule obtained by x-ray analysis . In: Nature . 181, No. 4610, March 1958, pp. 662-6. doi : 10.1038 / 181662a0 . PMID 13517261 .
- ↑ MF Perutz, MG Rossmann, AF Cullis, H. Muirhead, G. Will, ACT North: Structure of H . In: Nature . 185, No. 4711, 1960, pp. 416-422. doi : 10.1038 / 185416a0 . PMID 18990801 .
- ↑ Free State of Saxony LfULG series of publications, issue 35/2009 "Iron content of meat"
- ↑ Kristian Thygesen, Joseph S. Alpert, Allan S. Jaffe, Maarten L. Simoons, Bernard R. Chaitman: Third Universal Definition of Myocardial Infarction . In: Journal of the American College of Cardiology . tape 60 , no. 16 , p. 1581–1598 , doi : 10.1016 / j.jacc.2012.08.001 ( elsevier.com [accessed July 9, 2017]).
- ^ Daniel P. Runde: Injuries caused by electricity - injuries, poisoning. April 2018, accessed April 6, 2020 .
- ↑ Stone MJ, Waterman MR, Harimoto D, et al. : Serum myoglobin level as diagnostic test in patients with acute myocardial infarction . In: British Heart Journal . 39, No. 4, April 1977, pp. 375-80. PMID 869974 . PMC 483248 (free full text).
- ↑ Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB: Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney's mitochondria . In: Biochim. Biophys. Acta . 1792, No. 8, August 2009, pp. 796-803. doi : 10.1016 / j.bbadis.2009.06.005 . PMID 19545623 .
- ↑ laboratory lexicon: myoglobin