Tertiary structure
In biochemistry, tertiary structure is understood to be the superordinate spatial structure of proteins , nucleic acids or other macromolecules that consist of a single chain. It is composed of several elements of the secondary structure and, just like the secondary structure, is already coded in the primary structure .
The quaternary structure is superordinate to the tertiary structure . The hierarchical division into primary structure, secondary structure, tertiary structure and quaternary structure was proposed in 1952 by Kaj Ulrik Linderstrøm-Lang .
Proteins
In the case of proteins in particular, the three-dimensional structure is characteristic and essential for biological function. During or after the production of the protein by translation of an mRNA , the protein is converted into the biologically effective form by protein folding and passes through the cell again. This process is u. a. supported by chaperones .
In the case of a globular protein, the energetic driving force for the folding of the individual secondary structural elements is described by the Kauzmann rule : the hydrophobic areas are inside, while the hydrophilic and / or charged areas face the aqueous environment (see also hydrophobic effect ). The secondary structures are assembled into defined elements. Well-known protein folding classes are, for example, the 3- or 4- helix bundle , the β-barrel , the β-sandwich or the TIM-barrel .
Disulfide bonds (strongest bond), ionic bonds , hydrogen bonds and hydrophobic interactions (weakest bond) are involved in the stabilization of tertiary structures .
The next higher level is the quaternary structure .
Nucleic acids
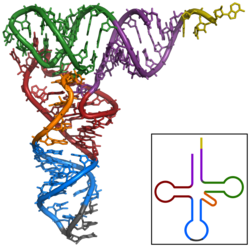
Nucleic acids can also adopt complex spatial structures: tRNAs must be in the correct tertiary structure for their function.
Tertiary structure of a pseudoknot
Structure elucidation
The area of biochemistry that deals with the elucidation or the effects of such structures is called structural biology . The methods of structure elucidation are mainly (X-ray) crystal structure analysis and multi-dimensional NMR . The bioinformatics developed methods or algorithms for predicting the three-dimensional structure of the protein from its amino acid sequence ( primary structure ).
Individual evidence
- ↑ Entry on tertiary structure . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.T06282 .