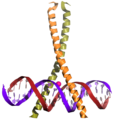
Coiled coil

The English term coiled coil , roughly translated twisted screw or double helix , describes a protein structure motif. A double helix is a helix, which in turn is wound into a helix with a larger radius.
A coiled coil is a stable, linear domain that consists of at least two single helices. As a rule, these are α-helices . These single helices are wound around one another to form a multiple helix. Accordingly, each of the twisted individual helices is a double helix. The term as such does not include the fact that a coiled coil consists of several individual helices.
The so-called heptad pattern is characteristic of coiled coils , in which hydrophobic amino acid residues are in the 1st and 4th position of a section comprising seven amino acids. The coiled coil structure is stabilized by the hydrophobic interaction of these amino acids.
One of the most common coiled coil structures is the bZIP domain ( also known as the leucine zipper).
Coiled coil motifs can be found in many proteins, for example in transcription factors or in proteins involved in vesicle transport. Presumably, they often continue to function as spacers. For some proteins, they also contribute to the dimer in formation.
Leucine zipper domain of the transcription factor GCN4
The transcription factor CREB
Coiled-coil proteins
- Lamin and other intermediate filaments
- Certain myosins
- fibrin
literature
- Alison K. Gillingham, Sean Munro: Long coiled-coil proteins and membrane traffic. In: Biochimica et Biophysica Acta . Vol. 1641, No. 2/3, 2003, pp. 71-85, doi : 10.1016 / S0167-4889 (03) 00088-0 .
- Y. Bruce Yu: Coiled-coils: stability, specificity, and drug delivery potential. In: Advanced Drug Delivery Reviews . Vol. 54, No. 8, 2002, pp. 1113-1129, doi : 10.1016 / S0169-409X (02) 00058-3 .