Myosin
Myosin (from ancient Greek μυός myos , genitive to μῦς mys 'muscle') refers to a family of motor proteins in eukaryotic cells . As an essential component in muscle , myosin is also involved in the conversion of chemical energy into strength and movement. In cooperation with other motor proteins such as kinesin and dynein , it is also involved in the intracellular transport of biomacromolecules, vesicles and cell organelles along the structures of the cytoskeleton . In contrast to kinesin and dynein, myosin moves along actin filaments . Other cellular functions include cell movement and adhesion , among others .
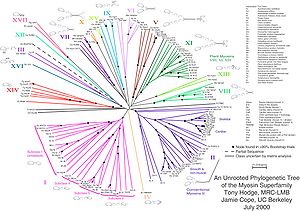
Myosin classes
First, myosin was identified as a motor protein of muscle fibers (part of the sarcomere , the contractible sections of the myofibrils (muscle fibrils)), whereby a bundle of myofibrils forms a muscle fiber. Later other members of the protein family appeared, the function of which is still largely unknown. For some of these unconventional myosins, functions in the intracellular transport of vesicles and organelles, in endocytosis or cell growth could be identified.
According to phylogenetic studies (see homology (biology) ), the myosin family is divided into classes and subclasses. Myosin found in muscle fibers belongs to class II with some other non-muscle myosins, which are also known as conventional myosins. All other classes are known as unconventional myosins. The unconventional myosins discovered first were grouped in Class I. Newer classes of unconventional myosins are numbered consecutively (III, IV, V…). 18 classes of unconventional myosins have been officially named, with at least six other, not yet named classes known. However, some delimitations and classifications are questionable.
Protein structure
Functional myosin consists of several amino acid chains:
- a heavy chain as well
- a different number of light chains.
The myosin II molecule is available as a so-called dimer, which consists of a total of 6 subunits (hexamer):
- 2 heavy chains
- 4 light chains
Heavy chain ( Heavy Chain )
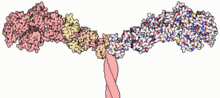
Common to the heavy chains of all myosins is a conserved head domain that combines the catalytic ATPase properties and is therefore also called the motor domain .
This is followed by the neck region, which can contain a different number of binding domains for light chains ( Light Chain Binding Domain , LCBD). An example of this is the IQ motif (consensus sequence: IQXXXRXXXXR), to which the protein calmodulin can bind in its function as a light chain. The number of LCBDs varies depending on the class. If coiled-coil structures are also formed, there is the possibility of dimerization, so that double-headed myosins arise.
The following tail region is also class-specific and shows the greatest differences between the different classes. This region often contains different protein interaction domains to which the cargo to be transported can dock. Conventional myosins are also known to have the tendency of the tail region of these dimeric molecules to form filaments. This is how myosin fibers form, which are part of the sarcomere . For non-muscle myosins it is assumed that the specificity of the transporter is determined via the domains in the tail region.
Light chain ( light chain )
The light chains are smaller amino acid chains that bind to the significantly larger heavy chain. The respective binding occurs specifically to binding domains for light chains (LCBD).
There are various light chains whose function is on the one hand purely structural (essential light chain) and on the other hand regulates the activity of the motor domain (regulatory light chain).
In particular, attention should be drawn to the regulation of muscle activity by Ca 2+ ions, which takes place via the regulatory light chain.
The movement of myosin in the cross-bridge cycle
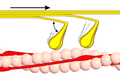
The example described here shows the movement of myosin using conventional myosin II in muscles, in which the actin and myosin filaments are pushed into one another. The process with the unconventional myosins is basically the same, except that here a myosin dimer with its cargo “wanders” along an actin filament. One myosin head binds alternately, so that “one foot at a time” is put forward. The movement is directed because myosin can only travel in one direction on the actin filament. Usually myosin runs towards the plus end of an actin filament. Myosin VI, which moves in the opposite direction, has so far been an exception. The different unconventional myosin classes take over the movement in both directions on the actin, in contrast to kinesin and dynein on the microtubules .
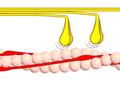
The motor activity is described by the so-called cross-bridge cycle. The motor domain is angled opposite the tail region. In the absence of ATP , it binds tightly to actin filaments. The binding of ATP causes it to dissociate from the actin filament. The angle of inclination of the motor domain then changes to around 90 ° (pre-power stroke conformation). After hydrolysis of ATP to ADP + P, myosin binds again to the actin filament. After releasing P and ADP, there is a power stroke, the tilt angle folding back to 50 ° (post-power stroke conformation). Since myosin is firmly bound to the actin filament again, a directed movement takes place. In the muscle, running through the cross-bridge cycle several times causes the myosin filaments and the actin filaments to slide into one another; muscle contraction occurs .
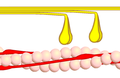
- Cross bridge cycle
Phase 1 - myosin (yellow) binds to actin (pink). This is made possible by conformational conversion of the tropomyosin, which thus releases the actin binding sites for myosin. This conformational change of tropomyosin is triggered by the binding of Ca 2+ ions to troponin. The indicated angle is about 90 °.
Provision of energy
The ATP supply in the muscle is usually sufficient for five to six seconds of continuous exercise. Then creatine phosphate (about ten more seconds is enough) and finally glucose (grape sugar) are metabolized by the muscles.
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6th edition, Spektrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 4th edition, John Wiley & Sons, New York 2011, ISBN 978-1-11813992-9 .
- Bruce Alberts , Alexander Johnson, Peter Walter, Julian Lewis, Martin Raff, Keith Roberts: Molecular Biology of the Cell , 4th Edition, Taylor & Francis 2002, ISBN 978-0-81533218-3 .
Web links
Individual evidence
- ↑ Renate Wahrig-Burfeind (Ed.): True. Illustrated dictionary of the German language . ADAC-Verlag, Munich 2004, ISBN 3-577-10051-6 , pp. 590 .